In Vitro Bioaccessibility of Citrus Pomace Compounds Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Methodology
2.2.1. Analysis of Individual Phenolic Compounds Composing Citrus Pomaces
2.2.2. Analysis of Bioaccessibility of Bioactive Compounds in Citrus Pomaces and Cookies
2.2.3. Assessment of Food Sensory Quality
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Polyphenol Profile of Citrus Pomaces
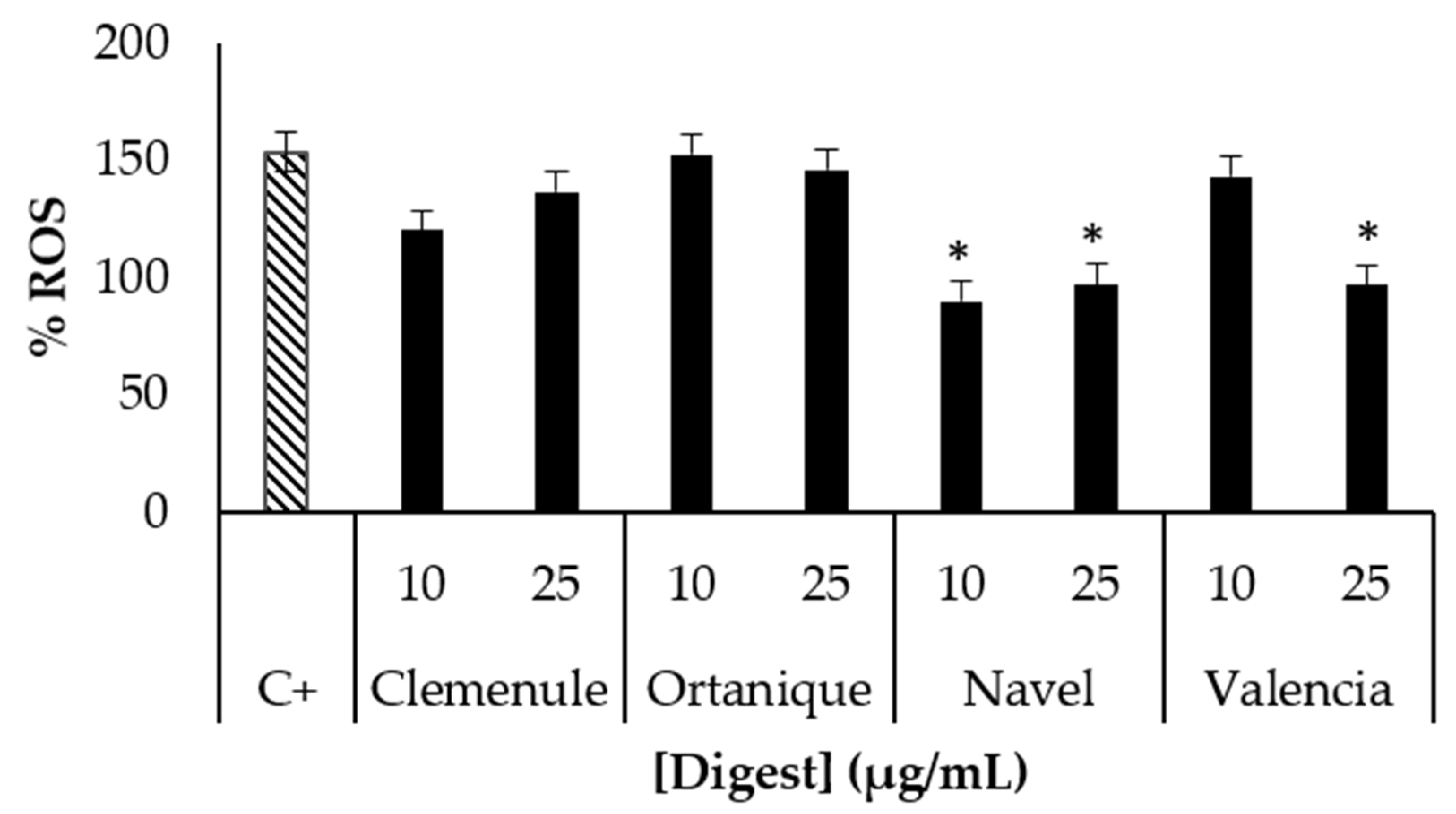
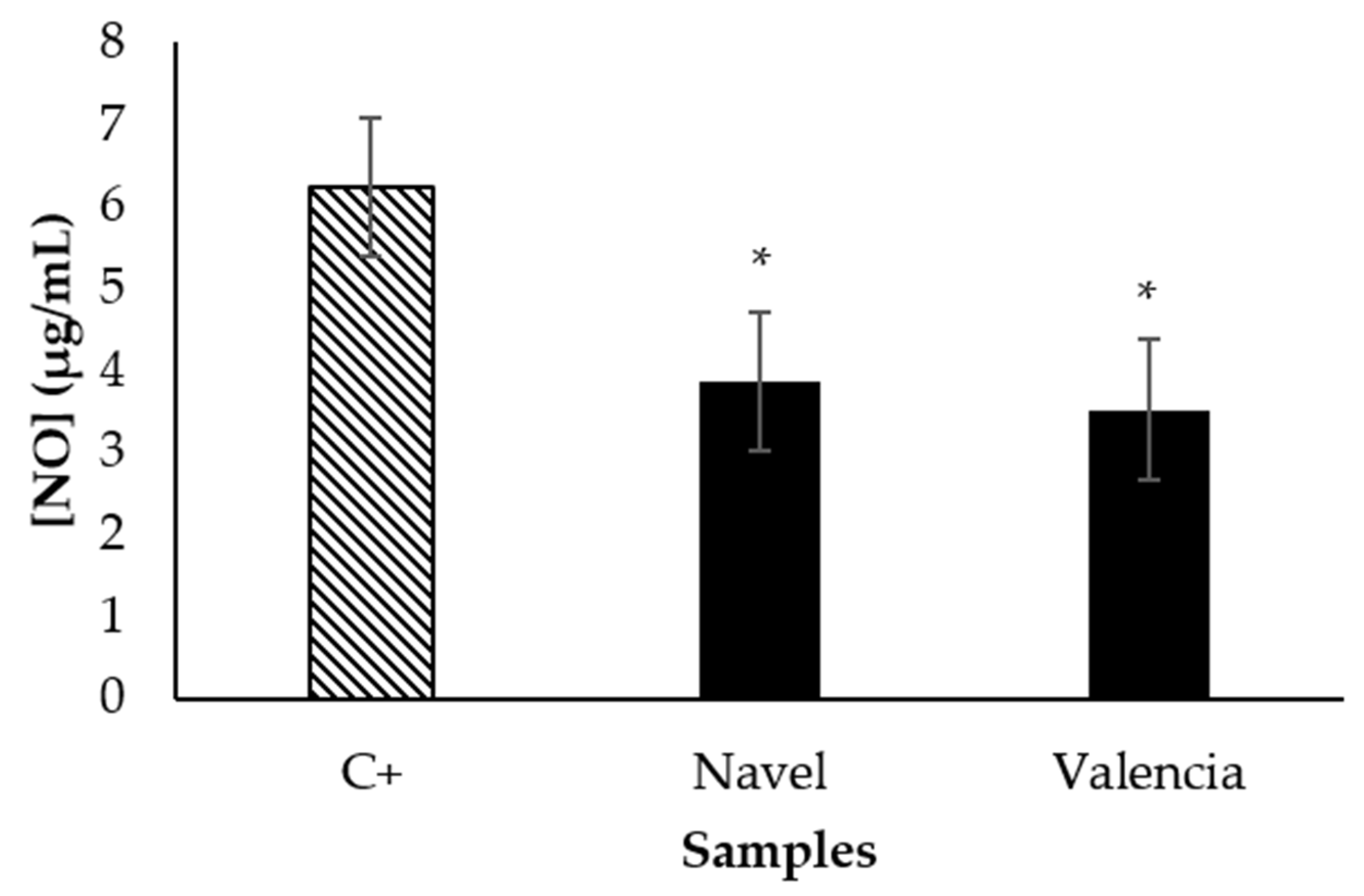
3.2. Bioaccessibility of Bioactive Compounds Composing Citrus Pomaces
3.3. Food Matrix Effect on the Bioaccessibility of Bioactive Compounds and Food Sensory Quality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 25 November 2019).
- Fernández-Fernández, A.M.; Dellacassa, E.; Medrano-Fernandez, A.; Del Castillo, M.D. Citrus Waste Recovery for Sustainable Nutrition and Health. In Food Wastes and By-Products: Nutraceutical and Health Potential; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 193–211. ISBN 9781119534105. [Google Scholar]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Cancalon, P.F.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br. J. Nutr. 2019, 121, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Li, S.; Liu, Z.; Tian, H.; Yin, X.; Huai, P.; Tang, W.; Zhou, D.; Steffen, L.M. Intake of fruit juice and incidence of type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2014, 9, e93471. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.K.; Riedl, K.M.; Cooperstone, J.L.; Högel, J.; Bosy-Westphal, A.; Schwartz, S.J.; Carle, R.; Schweiggert, R.M. Urinary excretion of Citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study. Mol. Nutr. Food Res. 2016, 60, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Dew, T.P.; Lévèques, A.; Pereira-Caro, G.; Rein, M.; Teml, A.; Schäfer, C.; Hofmann, U.; Schwab, M.; Eichelbaum, M.; et al. Gastrointestinal absorption and metabolism of hesperetin-7-O-rutinoside and hesperetin-7-O-glucoside in healthy humans. Mol. Nutr. Food Res. 2015, 59, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Hollebeeck, S.; Borlon, F.; Schneider, Y.-J.; Larondelle, Y.; Rogez, H. Development of a standardised human in vitro digestion protocol based on macronutrient digestion using response surface methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; Hochkogler, C.M.; Somoza, V.; del Castillo, M.D. Biscuits with no added sugar containing stevia, coffee fibre and fructooligosaccharides modifies α-glucosidase activity and the release of GLP-1 from HuTu-80 cells and serotonin from Caco-2 cells after in vitro digestion. Nutrients 2017, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Serra, M.; Larcher, R. Non-targeted glycosidic profiling of international wines using neutral loss-high resolution mass spectrometry. J. Chromatogr. A 2018, 1557, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of antioxidant, antidiabetic, antiobesity, and anti-inflammatory properties of a Tannat winemaking by-product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Ramírez, B.; Escobar, F.V.; del Dolores Castillo, M. Antioxidant properties of high molecular weight compounds from coffee roasting and brewing byproducts. Bioact. Compd. Health Dis. 2019, 2, 48–63. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon Tannin Decreased the Glycemic Response through Decreasing the Digestibility of Starch and Inhibiting α-Amylase, α-Glucosidase, and Intestinal Glucose Uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H2O2-induced oxidative stress in Caco-2 cells. Food Res. Int. 2018, 103, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-T.; Chu, H.-L.; Chyau, C.-C.; Chu, C.-C.; Duh, P.-D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Y.; Niu, X.; Tang, H.; Meydani, S.N.; Wu, D. Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmune inflammatory responses in mice. J. Nutr. Biochem. 2018, 54, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; Chen, K.Y.; Ho, C.-T.; Li, S. Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones. Food Sci. Hum. Wellness 2014, 3, 26–35. [Google Scholar] [CrossRef]
- Nakajima, V.M.; Moala, T.; Caria, C.R.P.; Moura, C.S.; Amaya-Farfan, J.; Gambero, A.; Macedo, G.A.; Macedo, J.A. Biotransformed citrus extract as a source of anti-inflammatory polyphenols: Effects in macrophages and adipocytes. Food Res. Int. 2017, 97, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, M.; Trabelsi, S.; Bejar, S. Citrus flavonoids collectively dominate the α-amylase and α-glucosidase inhibitions. Biologia 2017, 72, 764–773. [Google Scholar] [CrossRef]
| Analysis | Clemenule Mandarin | Ortanique Mandarin | Navel Orange | Valencia Orange |
|---|---|---|---|---|
| TPC (mg GAE/g sample) | 9.98 ± 0.51 b | 11.00 ± 0.65 b | 10.80 ± 1.67 b | 6.62 ± 0.38 a |
| ABTS (µmol TE/g sample) | 90.1 ± 1.5 b | 92.0 ± 2.0 bc | 65.5 ± 4.4 a | 97.1 ± 10.4 c |
| ORAC-FL (µmol TE/g sample) | 135.5 ± 22.2 a | 232.5 ± 26.7 b | 214.8 ± 20.0 b | 207.8 ± 29.0 b |
| Analysis | Clemenule Mandarin | Ortanique Mandarin | Navel Orange | Valencia Orange |
|---|---|---|---|---|
| α-glucosidase (IC50, mg/mL) | 3.97 ± 0.97 a | 4.93 ± 0.41 a | 11.42 ± 0.89 b | 5.09 ± 0.39 a |
| α-amylase (IC50, mg/mL) | 58.04 ± 2.09 a | 105.68 ± 16.03 b | 62.00 ± 1.62 a | 101.17 ± 4.70 b |
| Analysis | Control Cookie | Navel Cookie | Valencia Cookie |
|---|---|---|---|
| Overall antioxidant capacity | |||
| ABTS (µmol TE/g sample) | 82.36 ± 3.73 a | 99.30 ± 3.68 b | 100.81 ± 12.98 b |
| ORAC-FL (µmol TE/g sample) | 74.66 ± 9.09 a | 115.95 ± 21.73 b | 118.02 ± 6.41 b |
| Inhibition of carbohydrase activity | |||
| α-glucosidase (IC50, mg/mL) | 5.66 ± 0.61 b | 3.90 ± 0.20 a | 4.62 ± 0.43 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Gámbaro, A.; Medrano-Fernandez, A.; Castillo, M.D.d. In Vitro Bioaccessibility of Citrus Pomace Compounds Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Proceedings 2020, 61, 31. https://doi.org/10.3390/IECN2020-06999
Fernández-Fernández AM, Dellacassa E, Nardin T, Larcher R, Gámbaro A, Medrano-Fernandez A, Castillo MDd. In Vitro Bioaccessibility of Citrus Pomace Compounds Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Proceedings. 2020; 61(1):31. https://doi.org/10.3390/IECN2020-06999
Chicago/Turabian StyleFernández-Fernández, Adriana Maite, Eduardo Dellacassa, Tiziana Nardin, Roberto Larcher, Adriana Gámbaro, Alejandra Medrano-Fernandez, and María Dolores del Castillo. 2020. "In Vitro Bioaccessibility of Citrus Pomace Compounds Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes" Proceedings 61, no. 1: 31. https://doi.org/10.3390/IECN2020-06999
APA StyleFernández-Fernández, A. M., Dellacassa, E., Nardin, T., Larcher, R., Gámbaro, A., Medrano-Fernandez, A., & Castillo, M. D. d. (2020). In Vitro Bioaccessibility of Citrus Pomace Compounds Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Proceedings, 61(1), 31. https://doi.org/10.3390/IECN2020-06999








