Identification of Antihypertensive Tripeptides in the Culture Medium of Lactobacillus helveticus D75 and D76 Strains †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Bacteria
2.2. Preparation of Samples for Chromatographic Analysis
2.3. MALDI-Mass Spectrometry
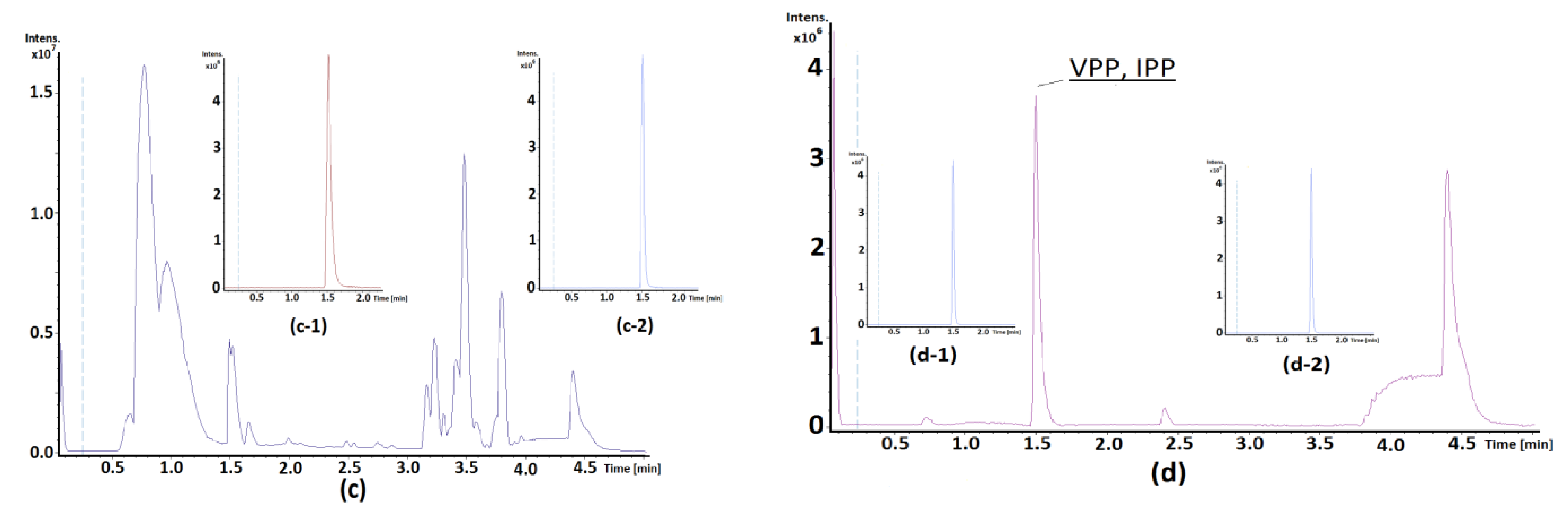
2.4. HPLC Analysis
2.5. HPLC-MS
3. Results and Discussion
References
- Vorob’ev, A.A.; Gershanovich, M.L.; Petrov, L.N. Prerequisites and perspectives for use of probiotics in complex therapy of cancer. Vopr. Onkol. 2004, 50, 361–365. [Google Scholar] [PubMed]
- Toropov, V.; Demyanova, E.; Shalaeva, O.; Sitkin, S.; Vakhitov, T. Whole-Genome Sequencing of Lactobacillus helveticus D75 and D76 Confirms Safety and Probiotic Potential. Microorganisms 2020, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Avalueva, E.B.; Sitkin, S.I.; Tkachenko, E.I.; Uspenskij, J.P.; Shevjakov, M.A.; Dobritsa, V.P.; Zhigalova, T.N.; Skazyvaeva, E.V.; Nilova, L.J.; Petrov, L.N. Method of Treating Patients with Ulcerative Colitis. Russian Patent RU 2419443, 27 May 2011. [Google Scholar]
- Jäkälä, P.; Vapaatalo, H. Antihypertensive Peptides from Milk Proteins. Pharmaceuticals 2010, 3, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Maeno, M.; Takano, T. Purification and characterization of an antihypertensive peptide from a yogurt-like product fermented by Lactobacillus helveticus CPN4. J. Dairy Sci. 1999, 82, 1388–1393. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. Health-Promoting Properties of Lactobacillus helveticus. Front. Microbiol. 2012, 3, 392. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Colletti, A.; Rosticci, M.; Cagnati, M.; Urso, R.; Giovannini, M.; Borghi, C.; D’Addato, S. Effect of Lactotripeptides (Isoleucine-Proline-Proline/Valine-Proline-Proline) on Blood Pressure and Arterial Stiffness Changes in Subjects with Suboptimal Blood Pressure Control and Metabolic Syndrome: A Double-Blind, Randomized, Crossover Clinical Trial. Metab. Syndr. Relat. Disord. 2016, 14, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Boelsma, E.; Kloek, J. IPP-rich milk protein hydrolysate lowers blood pressure in subjects with stage 1 hypertension, a randomized controlled trial. Nutr. J. 2010, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Aubin, F.; Azais-Braesco, V.; Borghi, C. Do the lactotripeptides isoleucine-proline-proline and valine-proline-proline reduce systolic blood pressure in European subjects? A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2013, 26, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U. The burden of hypertension. EuroIntervention 2013, 9, R12–R15. [Google Scholar] [CrossRef] [PubMed]
- Ozemek, C.; Laddu, D.R.; Arena, R.; Lavie, C.J. The role of diet for prevention and management of hypertension. Curr. Opin. Cardiol. 2018, 33, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Wu, J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) promote adipocyte differentiation and inhibit inflammation in 3T3-F442A cells. PLoS ONE 2015, 10, e0117492. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Jahandideh, F.; Davidge, S.T.; Wu, J. Milk-Derived Tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) Enhance Insulin Sensitivity and Prevent Insulin Resistance in 3T3-F442A Preadipocytes. J. Agric. Food Chem. 2018, 66, 10179–10187. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Cabello, I.; Herrera, M.O.; Artacho, R. Possible role of milk-derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr. Rev. 2012, 70, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Vakhitov, T.Y.; Chalisova, N.I.; Sitkin, S.I.; Sall, T.S.; Shalaeva, O.N.; Demyanova, E.V.; Morugina, A.S.; Vinogradova, A.F.; Petrov, A.V.; Nozdrachev, A.D. Low-molecular-weight components of the metabolome control the proliferative activity in cellular and bacterial cultures. Dokl. Biol. Sci. 2017, 472, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Falasconi, I.; Molinari, P.; Treu, L.; Basile, A.; Vezzi, A.; Campanaro, S.; Morelli, L. Genomic Comparison of Lactobacillus helveticus Strains Highlights Probiotic Potential. Front. Microbiol. 2019, 10, 1380. [Google Scholar] [CrossRef] [PubMed]



| t, min | 0.1% of Formic Acid | 100% ACN |
|---|---|---|
| 0 → 1.5 | 85% | 15% |
| 1.5 → 2.5 | 85% → 65% | 15% → 35% |
| 2.5 → 2.7 | 65% → 20% | 35% → 80% |
| 2.7 → 3.3 | 20% | 80% |
| 3.3→ 3.5 | 20% → 85% | 80% → 15% |
| 3.5 → 5 | 85% | 15% |
| Sample | Retention Time, min | Concentration, µg/mL | ||
|---|---|---|---|---|
| VPP | IPP | VPP | IPP | |
| D75 | 1.52 | 1.54 | 18.0 | 25.4 |
| D76 | 1.52 | 1.53 | 12.7 | 16.2 |
| D75 + D76 | 1.52 | 1.52 | 15.7 | 24.0 |
| IPP, VPP | 1.52 | 1.52 | 10.0 | 10.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuvakin, K.; Vakhitov, T.; Sitkin, S.; Roshchina, E.; Lisitskaya, V.; Ivanchenko, O.; Dubrovskii, Y.; Demyanova, E. Identification of Antihypertensive Tripeptides in the Culture Medium of Lactobacillus helveticus D75 and D76 Strains. Proceedings 2020, 61, 24. https://doi.org/10.3390/IECN2020-07014
Kuvakin K, Vakhitov T, Sitkin S, Roshchina E, Lisitskaya V, Ivanchenko O, Dubrovskii Y, Demyanova E. Identification of Antihypertensive Tripeptides in the Culture Medium of Lactobacillus helveticus D75 and D76 Strains. Proceedings. 2020; 61(1):24. https://doi.org/10.3390/IECN2020-07014
Chicago/Turabian StyleKuvakin, Kirill, Timur Vakhitov, Stanislav Sitkin, Evgeniya Roshchina, Veronika Lisitskaya, Olga Ivanchenko, Yaroslav Dubrovskii, and Elena Demyanova. 2020. "Identification of Antihypertensive Tripeptides in the Culture Medium of Lactobacillus helveticus D75 and D76 Strains" Proceedings 61, no. 1: 24. https://doi.org/10.3390/IECN2020-07014
APA StyleKuvakin, K., Vakhitov, T., Sitkin, S., Roshchina, E., Lisitskaya, V., Ivanchenko, O., Dubrovskii, Y., & Demyanova, E. (2020). Identification of Antihypertensive Tripeptides in the Culture Medium of Lactobacillus helveticus D75 and D76 Strains. Proceedings, 61(1), 24. https://doi.org/10.3390/IECN2020-07014





