Quenching of Fluorescence Caused by Graphene Oxide as an Immunosensing Platform in a Microwell Plate Format †
Abstract
:1. Introduction
2. Materials and Methods
2.1. GO-Coated Microwell (GOµW) Plates
2.2. GOµWs-Based Immunoassay
2.3. Conjugation of QDs with Anti-PSA
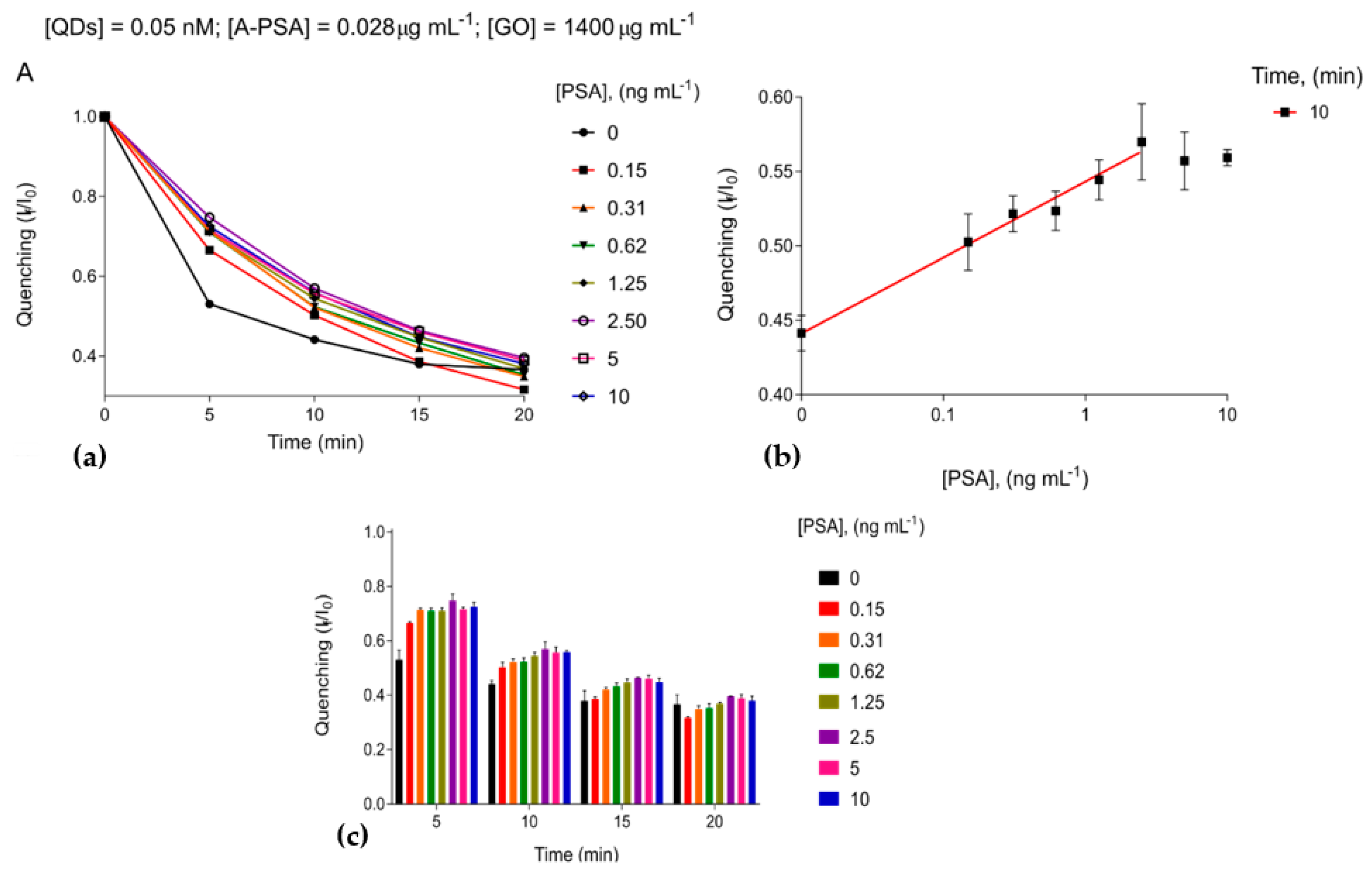
3. Results and Discussion
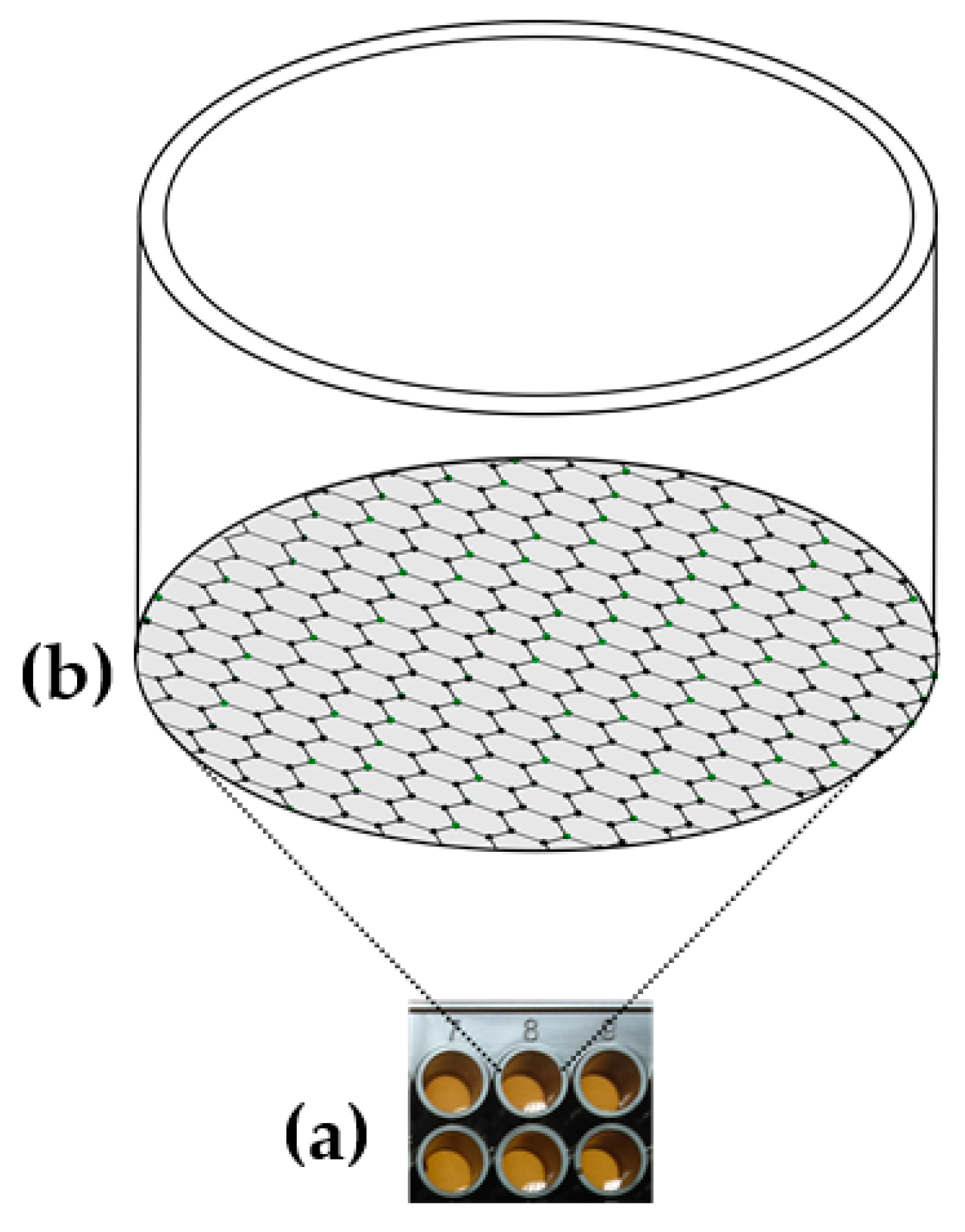
3.1. Characterization of Graphene Oxide-Coated Microwell Plates
3.2. The Immunosensing Platform Targeting Human IgG
3.3. The Immunosensing Platform Targeting Prostate-Specific Antigen
3.4. Proof of Concept of Immunosensing Platform
4. Conclusions
References
- Prasad, P.N. Introduction to Biophotonics; Chapter 9: Optical Biosensors; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 311–356. [Google Scholar]
- Morales-Narváez, E.; Merkoçi, A. Graphene Oxide as an Optical Biosensing Platform: A Progress Report. Adv. Mater. 2019, 31, 1805043. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Riaño, E.J.; Avila-Huerta, M.D.; Mancera-Zapata, D.L.; Morales-Narváez, E. Microwell plates coated with graphene oxide enable advantageous real-time immunosensing platform. Biosens. Bioelectron. 2020, 165, 112319. [Google Scholar] [CrossRef]
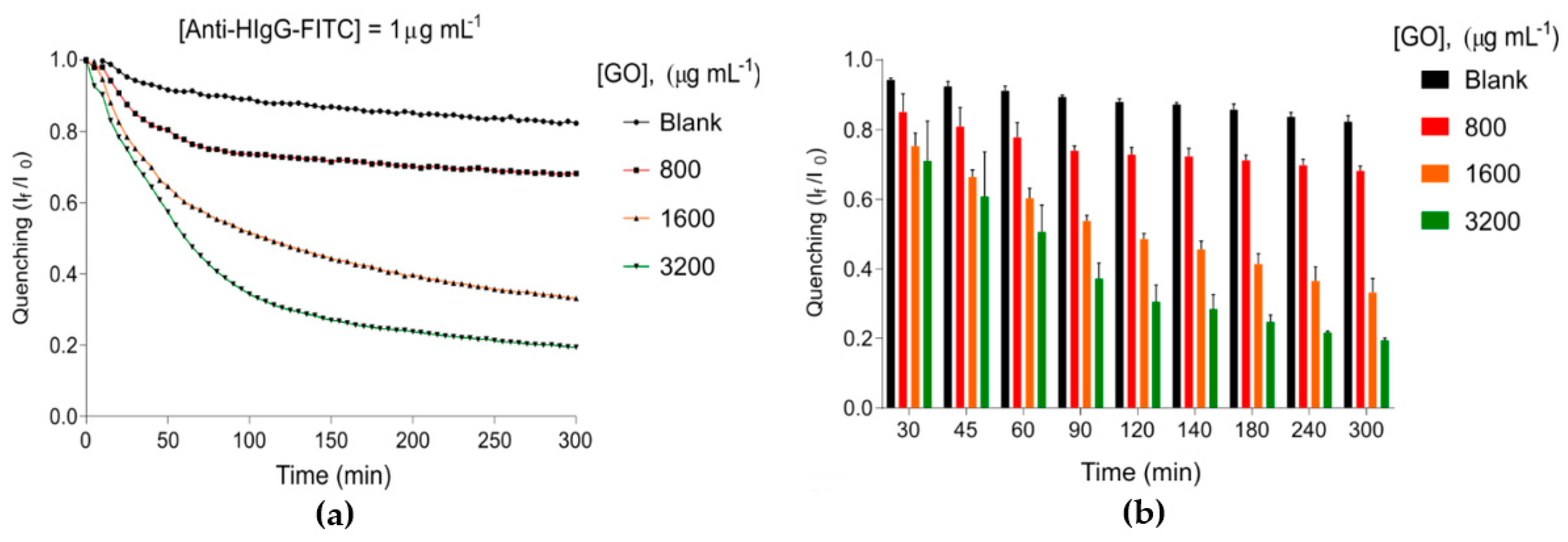
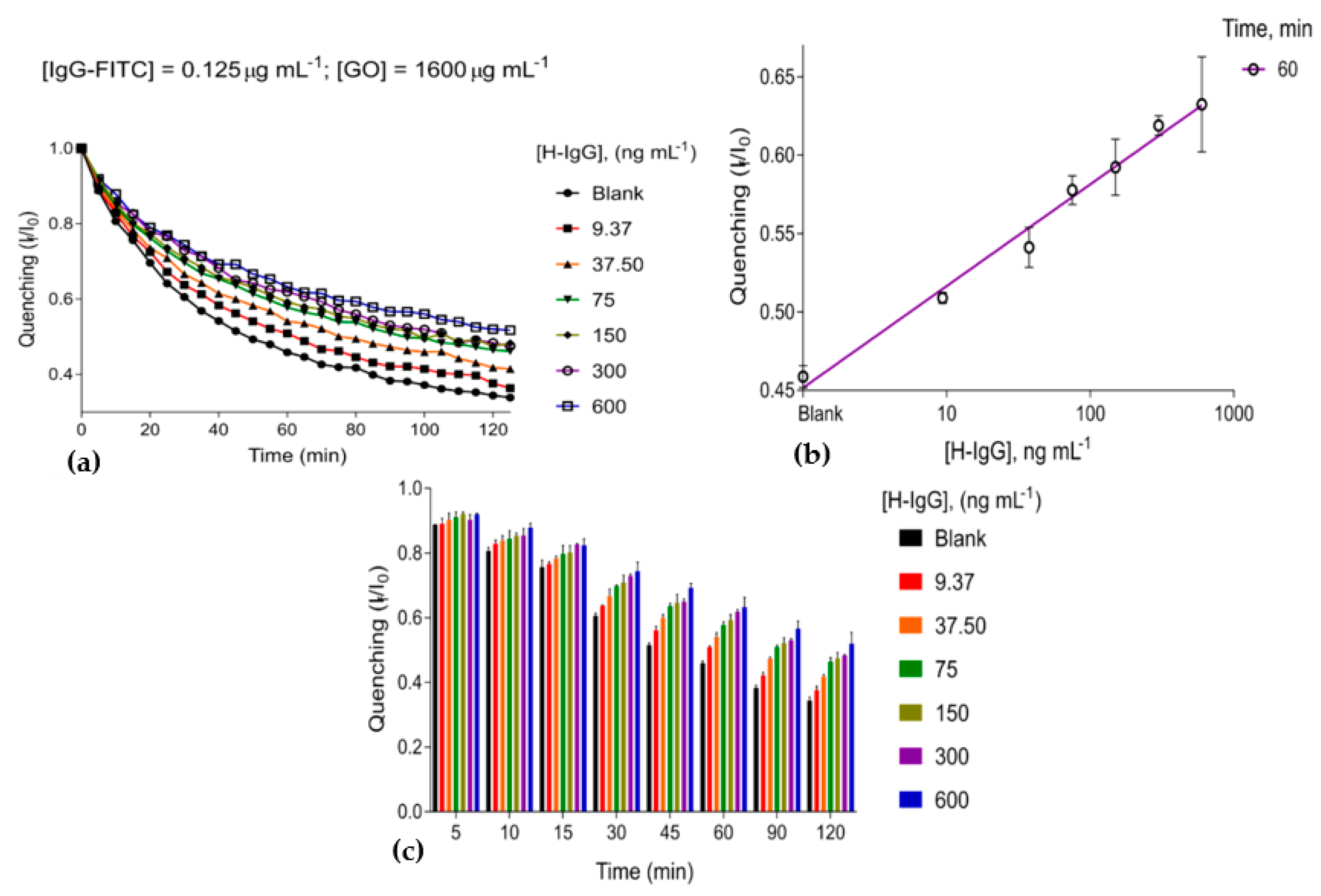
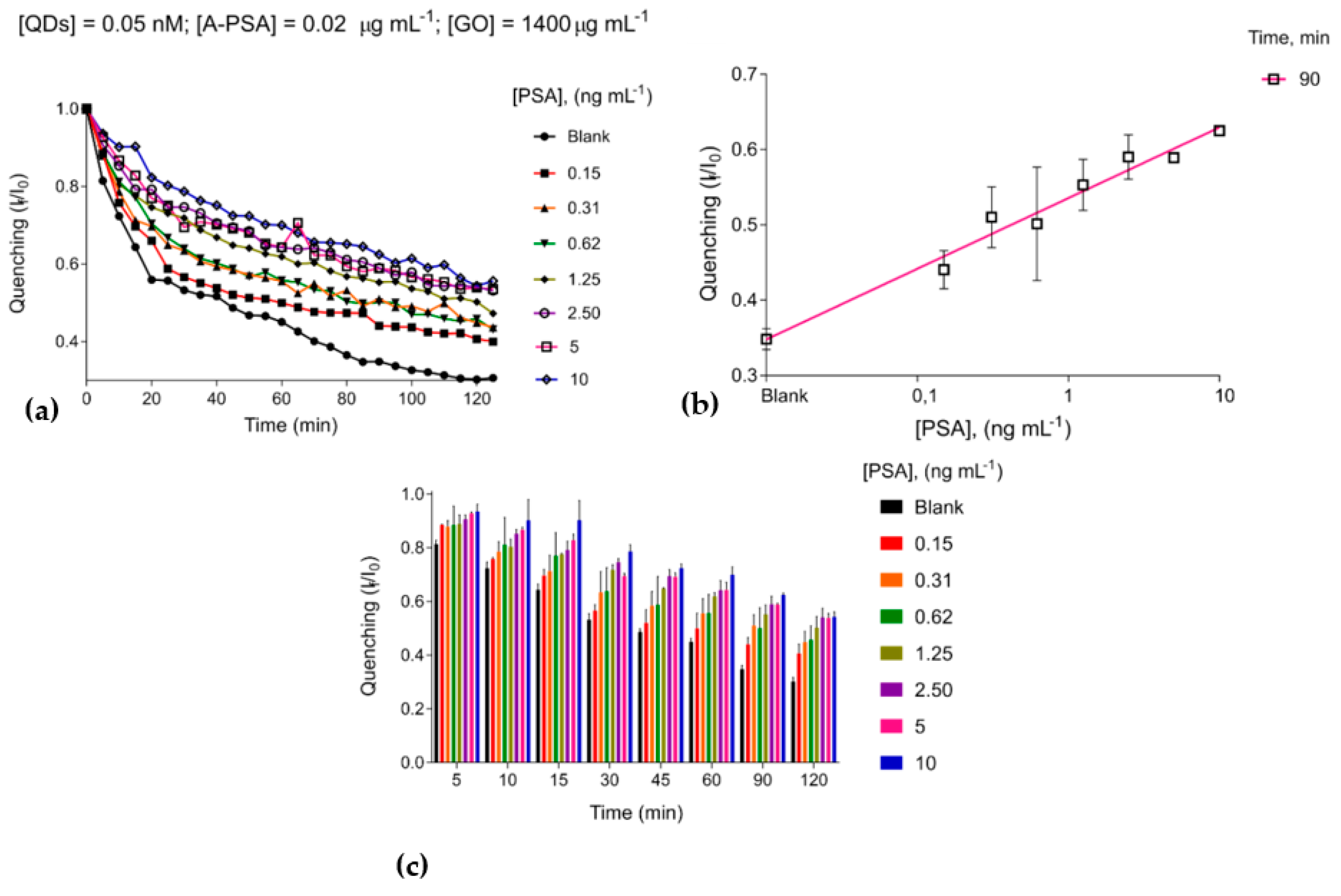
| Limit of Detection ng m | Optimal Concentration GO µg m | Optimal Concentration Anti-H-IgG µg m | Range of Coefficient of Variation (Precision) |
|---|---|---|---|
| 3.35 ± 1.08 | 1600 | 0.125 | 0.27–5% |
| Limit of Detection ng m | Optimal Concentration GO µg m | Optimal Concentration Qdot nM | Optimal Concentration Anti-PSA µg m | Range of Coefficient of Variation (Precision) |
|---|---|---|---|---|
| 0.02 | 1400 | 0.05 | 0.02 | 0.7–15.2% |
| Limit of Detection ng m | Optimal Concentration GO µg m | Optimal Concentration Qdot nM | Optimal Concentration Anti-PSA µg m | Range of Coefficient of Variation (Precision) |
|---|---|---|---|---|
| 0.05 | 1400 | 0.05 | 0.02 | 0.2–9.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Riaño, E.J.; Avila-Huerta, M.D.; Mancera-Zapata, D.L.; Morales-Narváez, E. Quenching of Fluorescence Caused by Graphene Oxide as an Immunosensing Platform in a Microwell Plate Format. Proceedings 2020, 60, 60. https://doi.org/10.3390/IECB2020-07017
Ortiz-Riaño EJ, Avila-Huerta MD, Mancera-Zapata DL, Morales-Narváez E. Quenching of Fluorescence Caused by Graphene Oxide as an Immunosensing Platform in a Microwell Plate Format. Proceedings. 2020; 60(1):60. https://doi.org/10.3390/IECB2020-07017
Chicago/Turabian StyleOrtiz-Riaño, Edwin J., Mariana D. Avila-Huerta, Diana L. Mancera-Zapata, and Eden Morales-Narváez. 2020. "Quenching of Fluorescence Caused by Graphene Oxide as an Immunosensing Platform in a Microwell Plate Format" Proceedings 60, no. 1: 60. https://doi.org/10.3390/IECB2020-07017
APA StyleOrtiz-Riaño, E. J., Avila-Huerta, M. D., Mancera-Zapata, D. L., & Morales-Narváez, E. (2020). Quenching of Fluorescence Caused by Graphene Oxide as an Immunosensing Platform in a Microwell Plate Format. Proceedings, 60(1), 60. https://doi.org/10.3390/IECB2020-07017






