Detection of Sub-Nanomolar Concentration of Trypsin by Thicken-Shear Mode (TSM) Acoustic Wave Biosensor †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cleaning and Modification of Gold Electrode-Coated Quartz Crystals
2.3. TSM Measurements
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gemene, K.L.; Meyerhoff, M.E. Detection of protease activities by flash chronopotentiometry using a reversible polycation-sensitive polymeric membrane electrode. Anal. Biochem. 2011, 416, 67–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siklos, M.; Aissa, B.; Thatcher, G.R.J. Cysteine proteases as therapeutic targets: Does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta Pharm. Sin. B 2015, 5, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.; Rosenlow, M.; Lindmark-Månsson, H.; Johansen, L.B.; Hartmann, J.; Hojer, A.; Waak, E.; Lofgren, R.; Saeden, K.H.; Svensson, S.; et al. Impact of protease and lipase activities on quality of Swedish raw milk. Int. Dairy J. 2020, 107, 104724. [Google Scholar] [CrossRef]
- Verdoes, M.; Verhels, S.H.L. Detection of protease activity in cells and animal. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 130–142. [Google Scholar] [CrossRef]
- Ku, M.; Hong, Y.; Heo, D.; Lee, E.; Hwang, S.; Suh, J.-S.; Yang, J. In vivo sensing of proteolytic activity with an NSET-based NIR fluorogenic nanosensor. Biosens. Bioelectr. 2016, 77, 471–477. [Google Scholar] [CrossRef]
- Buss, C.G.; Dudani, J.S.; Akana, R.T.K.; Fleming, H.E.; Bhatia, S.N. Protease activity sensors noninvasively classify bacterial infections and antibiotic responses. Ebiomedicine 2018, 38, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, T.; Qindeel, M.; Rehman, A.; Tarhini, M.; Ahmed, N.; Elaissari, A. Exploiting proteases for cancer theranostic through molecular imaging and drug delivery. Int. J. Pharm. 2020, 587, 119712. [Google Scholar] [CrossRef]
- Dunn, D.T.; Stöhr, W.; Arenas-Pinto, A.; Tostevin, A.; Mbisa, J.L.; Paton, N.I. Next generation sequencing of HIV-1 protease in the PIVOT trial of protease inhibitor monotherapy. J. Clin. Virol. 2018, 101, 63–65. [Google Scholar] [CrossRef]
- Kahler, U.; Kamenik, A.S.; Waibl, F.; Kraml, J.; Liedl, K.R. Protein-protein binding as a two-step mechanism: Preselection of encounter poses during the binding of BPTI and trypsin. Biophys. J. 2020, 119, 652–666. [Google Scholar] [CrossRef]
- Hou, S.; Feng, T.; Zhao, N.; Zhang, J.; Wang, H.; Liang, N.; Zhao, L. A carbon nanoparticle-peptide fluorescent sensor custom-made for simple and sensitive detection of trypsin. J. Pharm. Anal. 2020, in press. [Google Scholar] [CrossRef]
- Sato, D.; Kato, T. Novel fluorescent substrates for detection of trypsin activity and 541 inhibitor screening by self-quenching. Bioorg. Med. Chem. Lett. 2016, 26, 5736–5740. [Google Scholar] [CrossRef]
- Sao, K.; Murata, M.; Fujisaki, Y.; Umezaki, K.; Mori, T.; Niidome, T.; Katayama, Y.; Hashizume, M. A novel protease activity assay using a protease-responsive chaperone protein. Biochem. Biophys. Res. Commun. 2009, 383, 293–297. [Google Scholar] [CrossRef]
- Sakti, S.P.; Lucklum, R.; Hauptmann, P.; Bühling, F.; Ansorge, S. Disposable TSM-biosensor based on viscosity changes of the contacting medium. Biosens. Bioelect. 2001, 16, 1101–1108. [Google Scholar] [CrossRef]
- Cavic, B.A.; Thompson, M. Interfacial nucleic acid chemistry studied by acoustic shear wave propagation. Anal. Chim. Acta 2002, 469, 101–113. [Google Scholar] [CrossRef]
- Poturnayova, A.; Karpisova, I.; Castillo, G.; Mezo, G.; Kocsis, L.; Csámpai, A.; Keresztes, Z.; Hianik, T. Detection of plasmin based on specific peptide substrate using acoustic transducer. Sens. Actuators. B Chem. 2016, 223, 591–598. [Google Scholar] [CrossRef]
- Liu, J.; Chen, D.; Wang, P.; Song, G.; Zhang, X.; Li, Z.; Wang, Y.; Wang, J.; Yang, J. A microfabricated thickness shear mode electroacoustic resonator for the label-free detection of cardiac troponin in serum. Talanta 2020, 2015, 120890. [Google Scholar] [CrossRef] [PubMed]
- Šnejdárková, M.; Poturnayová, A.; Rybár, P.; Lhoták, P.; Himl, M.; Flídrová, K.; Hianik, T. High sensitive calixarene-based sensor for detection of dopamine by electrochemical and acoustic methods. Bioelectrochemistry 2010, 80, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tatarko, M.; Muckley, E.S.; Subjakova, V.; Goswami, M.G.; Sumpter, B.G.; Hianik, T.; Ivanov, I.N. Machine learning enabled acoustic detection of sub-nanomolar concentration of trypsin and plasmin in solution. Sens. Actuators B Chem. 2018, 272, 282–288. [Google Scholar] [CrossRef]
- Ahmadab, A.; Moore, E. Electrochemical immunosensor modified with self-assembled monolayer of 11-mercaptoundecanoic acid on gold electrodes for detection of benzo[a]pyrene in water. Analyst 2012, 37, 5839–5844. [Google Scholar] [CrossRef] [PubMed]
- Huenerbein, A.; Schmelzer, C.E.H.; Neubert, R.H.H. Real-time monitoring of peptic and tryptic digestions of bovine -casein using quartz crystal microbalance. Anal. Chim. Acta. 2007, 584, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lin, C.; Tao, T.; Lin, F. The effect of various concentrations of papain on the properties and hydrolytic rates of -casein layers. Colloids Surf. B Biointerfaces 2013, 101, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.S.; Cros, L. Adsorption of β-lactoglobulin and β-casein to metal surfaces and their removal by a non-ionic surfactant, as monitored via a quartz crystal microbalance. Colloids Surf. B Biointerfaces 1998, 10, 227–241. [Google Scholar] [CrossRef]
- Ozeki, T.; Verma, V.; Uppalapati, M.; Suzuki, Y.; Nakamura, M.; Catchmark, J.M.; Hancock, W.O. Surface-bound casein modulates the adsorption and activity of kinesin on SiO2 surfaces. Biophys. J. 2009, 96, 3305–3318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goda, T.; Miyahara, Y. Interpretation of protein adsorption through its intrinsic electric charges: A comparative study using a field-effect transistor, surface plasmon resonance, and quartz crystal microbalance. Langmuir 2012, 28, 14730–14738. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fuentes, L.; Drummond, C.; Faraudo, J.; Bastos-González, D. Adsorption of milk proteins (β-casein and β-lactoglobulin) and BSA onto hydrophobic surfaces. Materials 2017, 10, 893. [Google Scholar] [CrossRef]
- Miodek, A.; Poturnayová, A.; Šnejdárková, M.; Hianik, T.; Korri-Youssoufi, H. Binding kinetics of human cellular prion detection by DNA aptamers immobilized on a conducting polypyrrole. Anal. Bioanal. Chem. 2013, 405, 2505–2514. [Google Scholar] [CrossRef]
- Románszki, L.; Tatarko, M.; Jiao, M.; Keresztes, Z.; Hianik, T.; Thompson, M. Casein probe–based fast plasmin determination in the picomolar range by an ultra-high frequency acoustic wave biosensor. Sens. Actuators B Chem. 2018, 275, 206–214. [Google Scholar] [CrossRef]
- Nylander, T.; Wahlgren, N.M. Competitive and sequential adsorption of β-casein and β-lactoglobulin on hydrophobic surfaces and the interfacial structure of β-casein. J. Colloid Interface Sci. 1994, 162, 151–162. [Google Scholar] [CrossRef]
- Nylander, T.; Tiberg, F.; Wahlgren, N.M. Evaluation of the structure of adsorbed layers of β-casein from ellipsometry and surface force measurements. Int. Dairy J. 1999, 9, 313–317. [Google Scholar] [CrossRef]
- Krisdhasima, V.; Vinaraphong, P.; McGuire, J. Adsorption kinetics and elutability of α-lactalbumin, β-casein, β-lactoglobulin, and bovine serum albumin at hydrophobic and hydrophilic interfaces. J. Colloid Interface Sci. 1993, 161, 325–334. [Google Scholar] [CrossRef]
- Kari, J.; Andersen, M.; Borch, K.; Westh, P. An inverse Michaelis-Menten approach for interfacial enzyme kinetics. Catalysis 2017, 7, 4904–4914. [Google Scholar] [CrossRef]
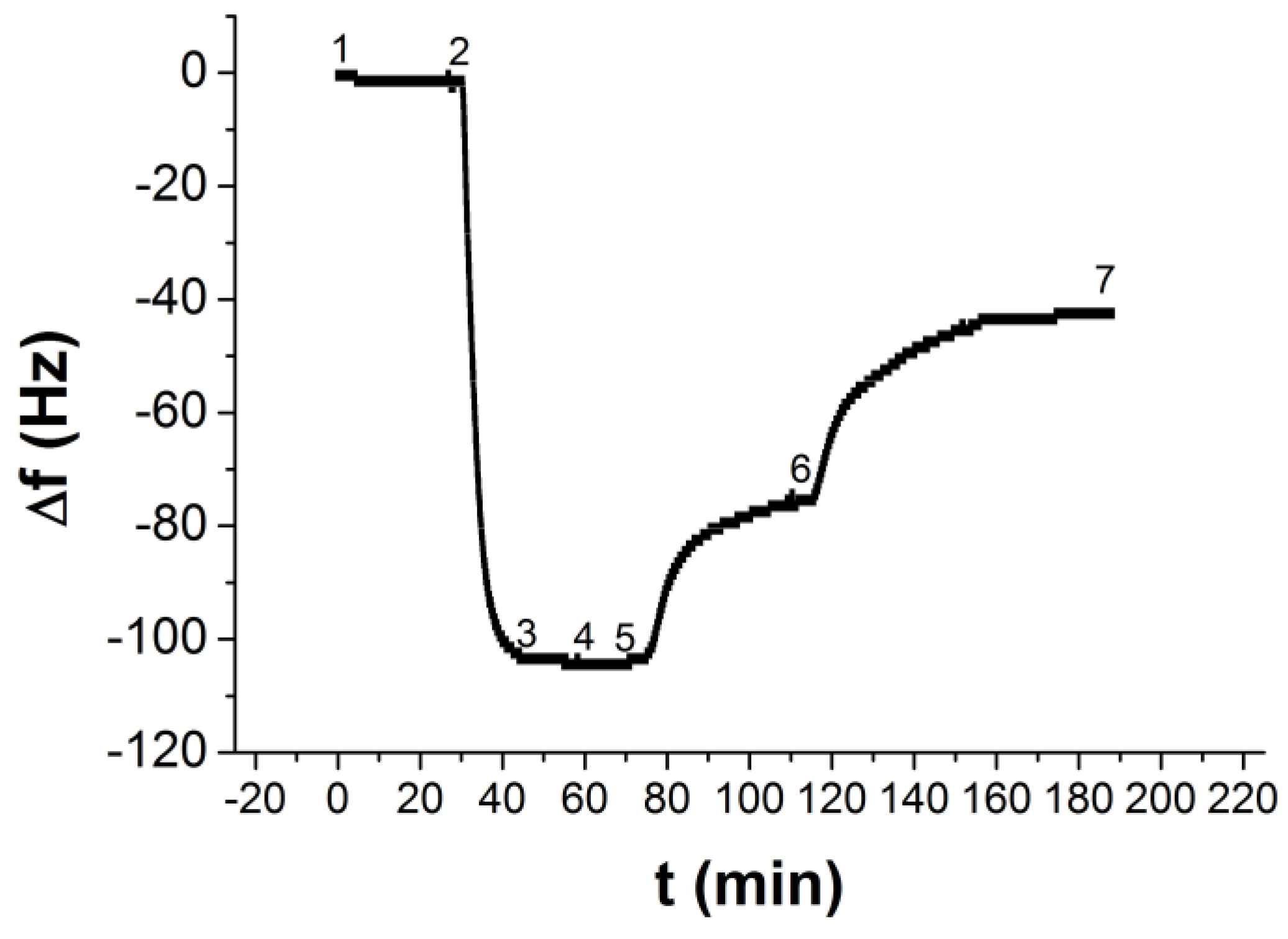
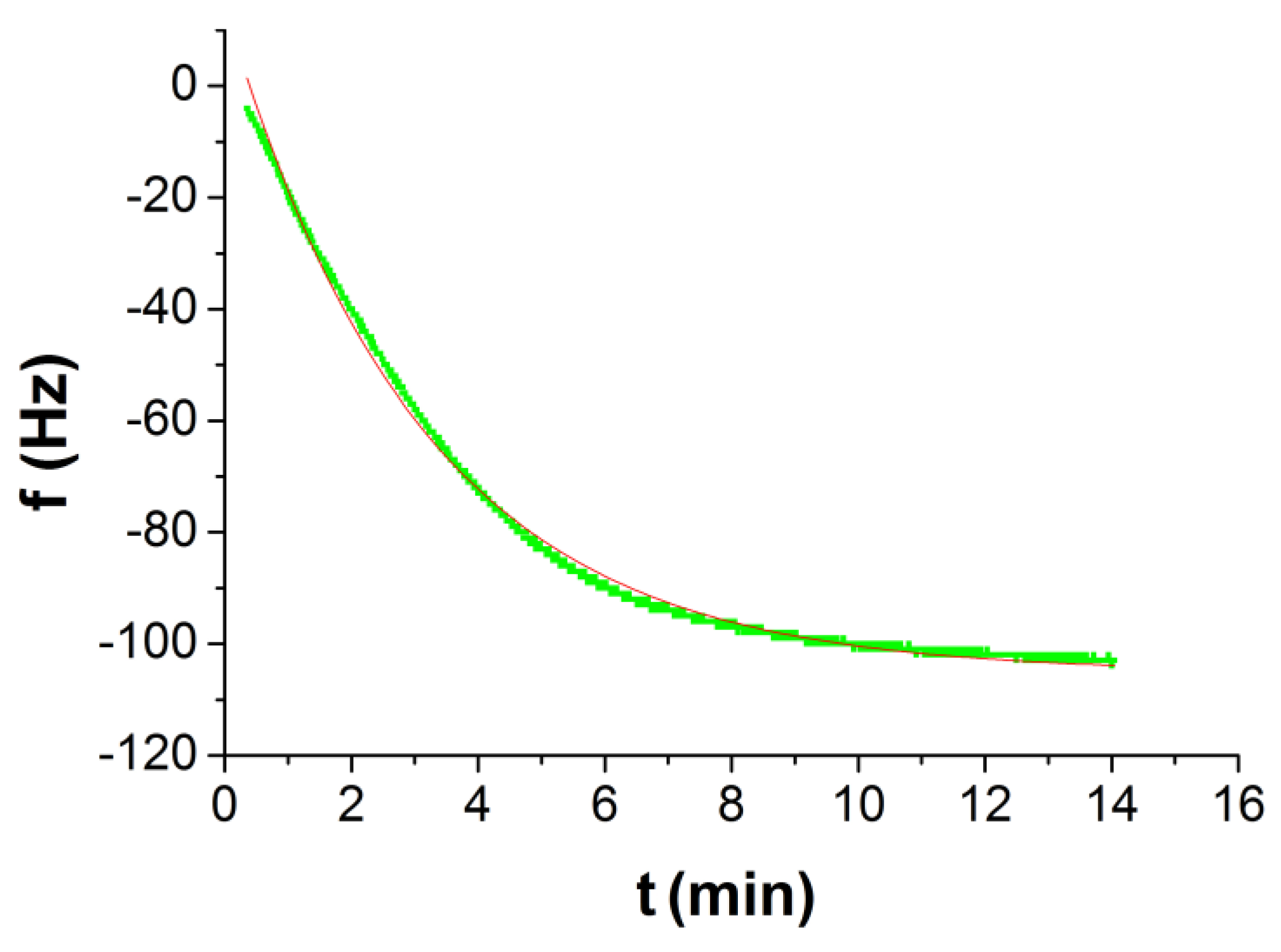

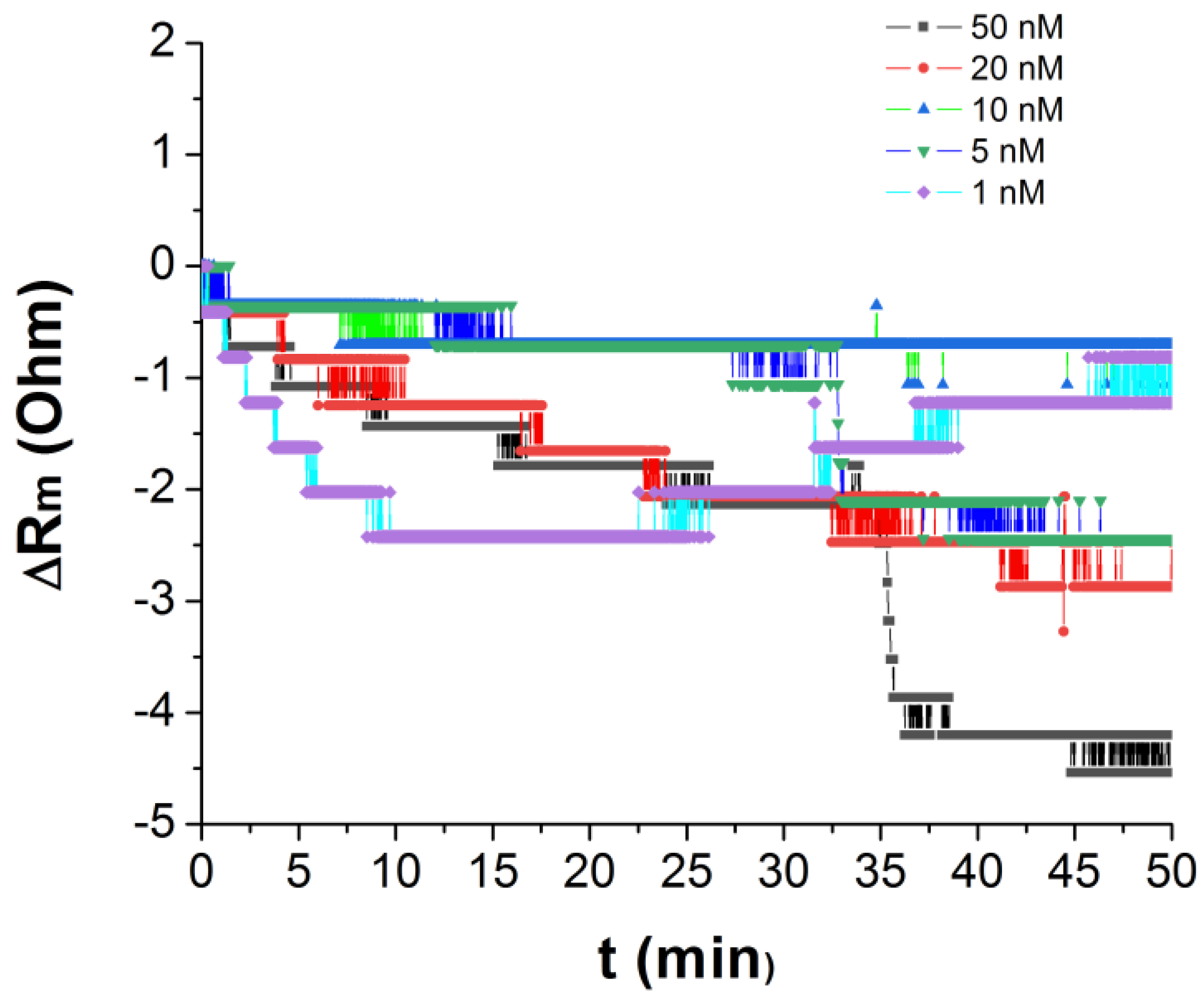
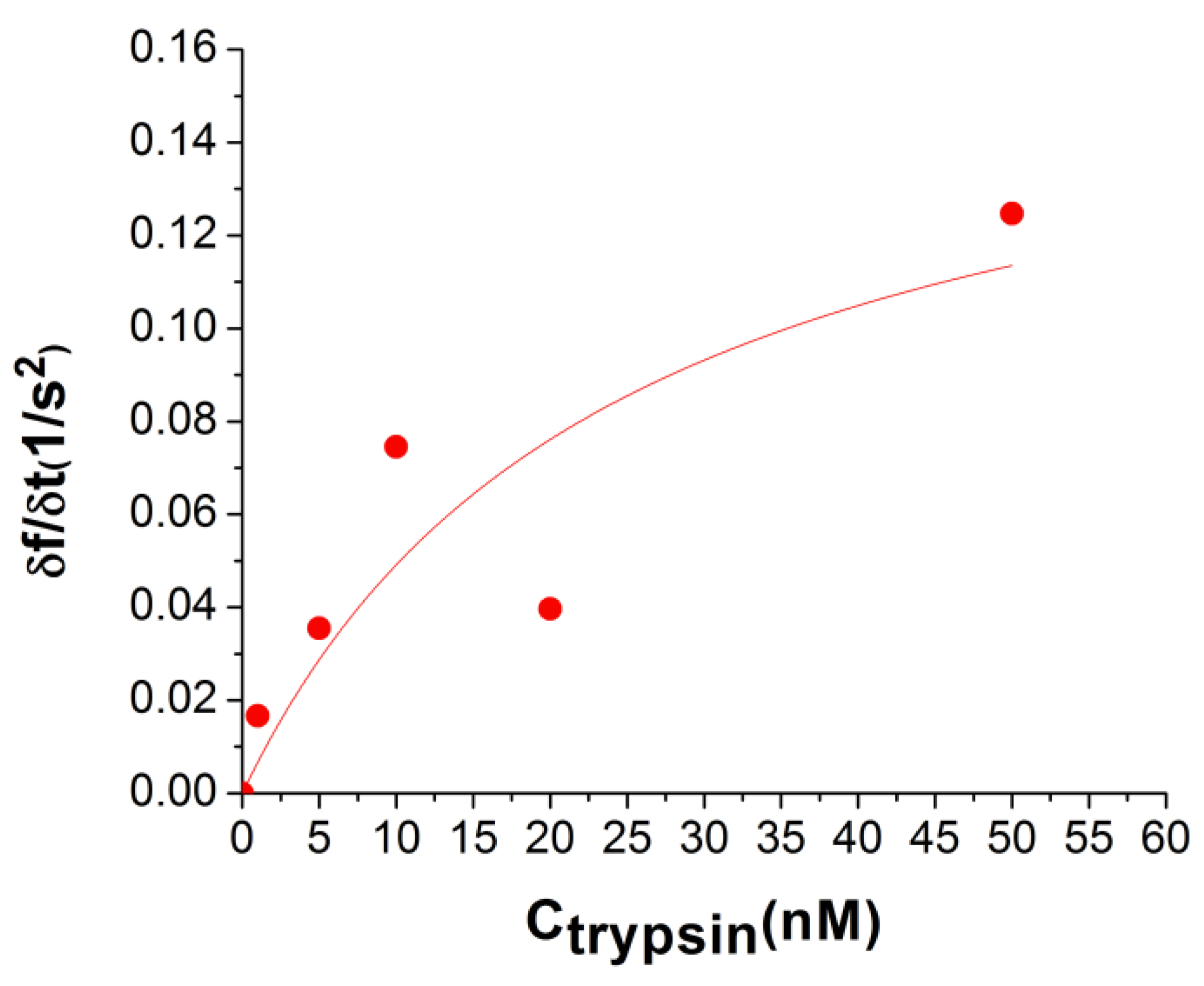
| τ1 (s) | Δf1 (Hz) | Δfn (Hz) |
|---|---|---|
| 186 | 119.32 | −105.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melikishvili, S.; Hianik, T.; Thompson, M. Detection of Sub-Nanomolar Concentration of Trypsin by Thicken-Shear Mode (TSM) Acoustic Wave Biosensor. Proceedings 2020, 60, 6. https://doi.org/10.3390/IECB2020-07075
Melikishvili S, Hianik T, Thompson M. Detection of Sub-Nanomolar Concentration of Trypsin by Thicken-Shear Mode (TSM) Acoustic Wave Biosensor. Proceedings. 2020; 60(1):6. https://doi.org/10.3390/IECB2020-07075
Chicago/Turabian StyleMelikishvili, Sopio, Tibor Hianik, and Michael Thompson. 2020. "Detection of Sub-Nanomolar Concentration of Trypsin by Thicken-Shear Mode (TSM) Acoustic Wave Biosensor" Proceedings 60, no. 1: 6. https://doi.org/10.3390/IECB2020-07075
APA StyleMelikishvili, S., Hianik, T., & Thompson, M. (2020). Detection of Sub-Nanomolar Concentration of Trypsin by Thicken-Shear Mode (TSM) Acoustic Wave Biosensor. Proceedings, 60(1), 6. https://doi.org/10.3390/IECB2020-07075







