Lab-on-a-Tip Based on a Bimetallic Nanoarchitecture Enabling Catalytic 4-Nitrophenol Switch-off †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Materials, Samples and Apparatus
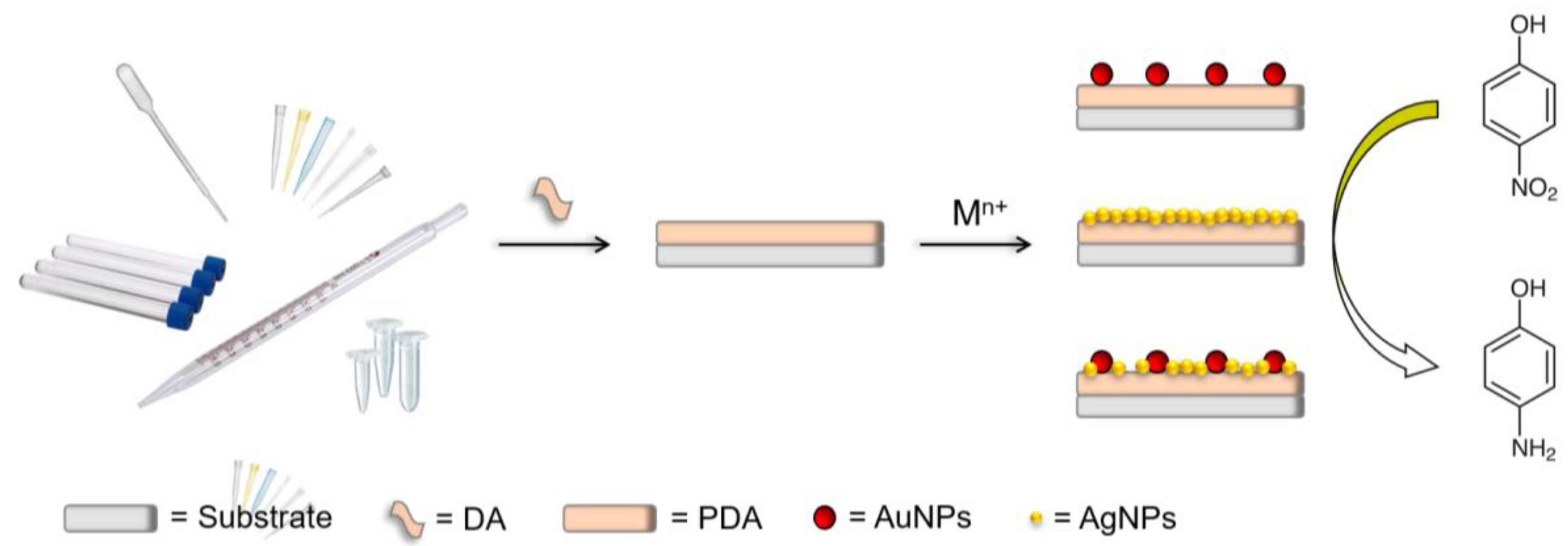
2.2. Metal Nanocomposite Fabrication in Laboratory-Tip
2.2.1. PDA Film Self-Assembly
2.2.2. Gold and Silver Nanodecoration
- AuNPs decoration: The PDA modified µ-Tips were filled with 800 μL of 100 μM Au(III) aqueous solution and incubated in the dark at room temperature for 8 h. The reaction was blocked emptying the Tips subsequently washed and rinsed with abundant Milli-Q water. The AuNPs decorated µ-T@PDA were let dry at room temperature.
- AgNPs decoration: The AuNPs-decorated µ-T@PDA (µ-T@Au) were filled with 740 μL of Milli-Q water and 40 μL of 20 mM AgNO3; afterward, 20 μL of 4 M NaOH was added to trig the reaction (final volume 800 μL). The reaction mix was orbitally shaken (SSL1, Stuart equipment, Belfast, UK150) at 150 rpm at room temperature, in the dark, for 4 h. The reaction was blocked, subsequently emptying the Tips, which were washed and rinsed with abundant Milli-Q water. The AgNPs decorated µ-T@Au (µ-T@Au@Ag) were left to dry at room temperature.
- Au@AgNPs decoration: For the bimetallic nanocomposite formation, the previous two steps were performed consecutively.
3. Results and Discussion
3.1. µ-Tip-Surfaces Nanodecoration
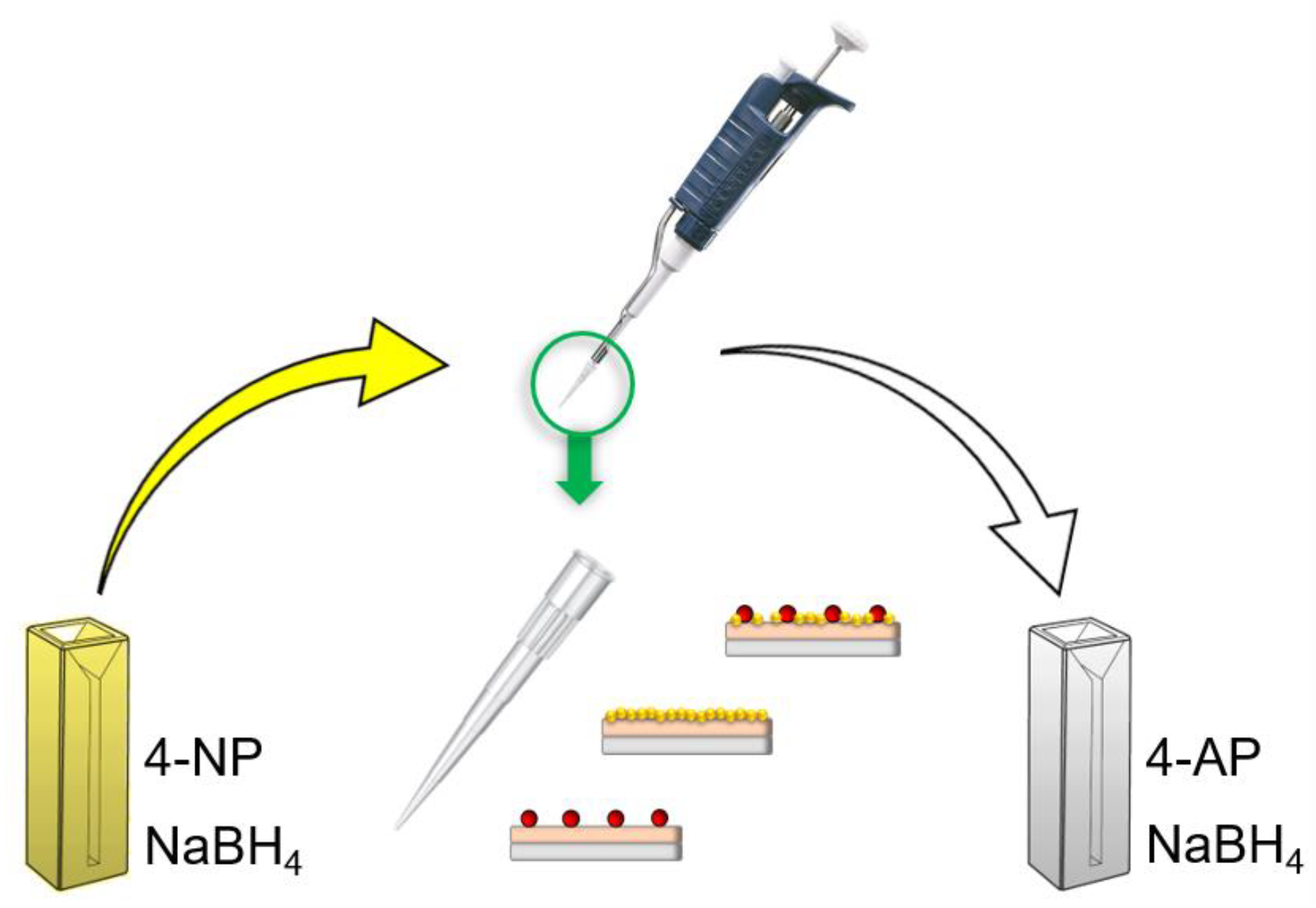
3.2. Reduction of 4-Nitrophenol by Using Nanodecorated µ-Tips
4. Conclusions
References
- Della Pelle, F.; Compagnone, D. Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Scroccarello, A.; Sergi, M.; Mascini, M.; Del Carlo, M.; Compagnone, D. Simple and rapid silver nanoparticles based antioxidant capacity assays: Reactivity study for phenolic compounds. Food Chem. 2018, 256, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Vilela, D.; Gonzàlez, M.C.; Lo Sterzo, C.; Compagnone, D.; Del Carlo, M.; Escarpa, A. Antioxidant capacity index based on gold nanoparticles formation. Application to extra virgin olive oil samples. Food Chem. 2015, 178, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Sergi, M.; Del Carlo, M.; Compagnone, D.; Escarpa, A. Gold Nanoparticles-based Extraction-Free Colorimetric Assay in Organic Media: An Optical Index for Determination of Total Polyphenols in Fat-Rich Samples. Anal. Chem. 2015, 87, 6905–6911. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Scroccarello, A.; Scarano, S.; Compagnone, D. Silver nanoparticles-based plasmonic assay for the determination of sugar content in food matrices. Anal. Chim. Acta 2019, 1051, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, N. Noble metal nanoparticles-based colorimetric biosensor for visual quantification: A mini review. Chemosensors 2019, 7, 53. [Google Scholar] [CrossRef]
- Berahim, N.; Basirun, W.; Leo, B.; Johan, M. Synthesis of Bimetallic Gold-Silver (Au-Ag) Nanoparticles for the Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol. Catalysts 2018, 8, 412. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, M.J.; Cha, S.H.; Kim, Y.S.; Cho, S.; Park, Y. Catechin-capped gold nanoparticles: Green synthesis, characterization, and catalytic activity toward 4-nitrophenol reduction. Nanoscale Res. Lett. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Hua, X.; Li, M.; Long, Y.T. Mussel-inspired polydopamine functionalized plasmonic nanocomposites for single-particle catalysis. ACS Appl. Mater. Interfaces 2017, 9, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, R.K. Work Function Based Catalytic Activity of Metallic Nanoparticles for Dye Degradation. Catal. Lett. 2019. [Google Scholar] [CrossRef]
- Baruah, D.; Goswami, M.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Biogenic synthesis of gold nanoparticles and their application in photocatalytic degradation of toxic dyes. J. Photochem. Photobiol. B Biol. 2018, 186, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kästner, C.; Thünemann, A.F. Catalytic Reduction of 4-Nitrophenol Using Silver Nanoparticles with Adjustable Activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Scroccarello, A.; Della Pelle, F.; Ferraro, G.; Fratini, E.; Tempera, F.; Dainese, E.; Compagnone, D. Plasmonic active film integrating gold/silver nanostructures for H2O2 readout. Talanta 2021, 222, 121682. [Google Scholar] [CrossRef] [PubMed]
- Scroccarello, A.; Della Pelle, F.; Fratini, E.; Ferraro, G.; Scarano, S.; Palladino, P.; Compagnone, D. Colorimetric determination of polyphenols via gold nanoseeds decorated polydopamine film. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]

| µ-Tip | Concentration Range (μM) | Linear Equation | Slope | R2 |
|---|---|---|---|---|
| µ-T@Au@Ag | 50–500 | y= −0.13 (0.00) x + 76.40 (0.30) 1 | −0.13 | 0.999 |
| µ-T@Ag | 50–500 | y= −0.11 (0.01) x + 59.47 (1.62) 1 | −0.11 | 0.998 |
| µ-T@Au | 50–500 | y= −0.04 (0.00) x + 19.18 (0.95) 1 | −0.04 | 0.985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scroccarello, A.; Pelle, F.D.; Compagnone, D. Lab-on-a-Tip Based on a Bimetallic Nanoarchitecture Enabling Catalytic 4-Nitrophenol Switch-off. Proceedings 2020, 60, 4. https://doi.org/10.3390/IECB2020-07083
Scroccarello A, Pelle FD, Compagnone D. Lab-on-a-Tip Based on a Bimetallic Nanoarchitecture Enabling Catalytic 4-Nitrophenol Switch-off. Proceedings. 2020; 60(1):4. https://doi.org/10.3390/IECB2020-07083
Chicago/Turabian StyleScroccarello, Annalisa, Flavio Della Pelle, and Dario Compagnone. 2020. "Lab-on-a-Tip Based on a Bimetallic Nanoarchitecture Enabling Catalytic 4-Nitrophenol Switch-off" Proceedings 60, no. 1: 4. https://doi.org/10.3390/IECB2020-07083
APA StyleScroccarello, A., Pelle, F. D., & Compagnone, D. (2020). Lab-on-a-Tip Based on a Bimetallic Nanoarchitecture Enabling Catalytic 4-Nitrophenol Switch-off. Proceedings, 60(1), 4. https://doi.org/10.3390/IECB2020-07083






