Abstract
Listeria monocytogenes is an intracellular bacterium that causes serious epidemic and sporadic food-borne illnesses in humans. Rapid and trustworthy methods are necessary for the detection of the pathogen to prevent potential food contamination. The aim of this study was to test a newly developed L. monocytogenes biosensor on actual food samples and validate its ability to detect the presence of pathogens robustly and accurately. The newly developed method uses a cell-based biosensor technology (BERA) and a portable device developed by EMBIO Diagnostics called B.EL.D, and provides results within 3 min. Tests were conducted on ready-to-eat lettuce salads, milk and halloumi cheese and the results indicate that the novel system was able to identify inoculated samples with 98%, 90%, and 91% accuracy, respectively. Furthermore, the limit of detection was determined to be as low as 0.6 log CFU mL−1 or g−1 in all food types. Classification of the samples Above or Below the detection limit was accessed through a newly developed algorithm for each food substrate. Samples were also analyzed with the ISO 11290-1:2017 and 11290-2:2017, in parallel. Thus, it was concluded that the newly developed biosensor can be a useful tool in the food supply chain, decreasing the required time for the detection of pathogens and increasing the number of tested samples before they reach the market.
1. Introduction
Foodborne diseases are defined by high incidence and low mortality rates. In the case of Listeria monocytogenes, however, the situation is different. Listeriosis is a relatively rare disease (0.1 to 10 cases per 1,000,000 people per year) with high hospitalization and mortality rates (90% and 20%–30%, respectively) [1]. These characteristics make this infection a significant public health concern and one of the most serious foodborne diseases under surveillance.
Human food-borne listeriosis is strongly linked to the consumption of poultry, beef, dairy products, and fresh produce. However, in the last decade, the majority of listeriosis infection has been mainly associated with dairy products and fresh produce consumption, resulting in hundreds of illnesses, hospitalizations, deaths, and product recalls [2]. Milk and dairy products are very good substrates for the growth of microorganisms, including pathogens, due to their high nutritional value. The main pathogens of concern in the dairy industry are the bacteria who can survive the manufacturing process and grow in low temperatures Therefore, L. monocytogenes, a psychrophile pathogenic bacterium capable of growing at refrigeration temperatures and surviving in freezing temperatures is one of the greatest concerns in the dairy industry. The pathogen is usually eliminated through milk pasteurization, but the level of destruction depends on the strain’s resistance and the pathogen’s population and may eventually result in its minimum survival [3,4]. This survival is crucial since it can lead to dangerous levels of multiplication despite the product’s proper refrigeration, indicating a significant risk for human infection, especially from dairy products produced by raw milk [5]. On the other hand, human listeriosis infections linked to fresh produce consumption have been significantly increased in the last two decades. This increase is mainly ascribed to consumers’ tendency to eat healthier by consuming higher amounts of fresh produce, and to the globalization of food supply that leads to the dissemination of pathogens over wide geographical areas. Furthermore, due to L. monocytogenes’ ubiquitous nature, fresh produce can be easily contaminated during harvest, and pre- and post-harvest processes [6].
The minimal infective dose for listeriosis is not precisely determined and it varies among individuals, based on their genetic predisposition and health condition [7]. Nevertheless, it is generally considered that 100–1000 CFU per gram of foodstuff are enough to cause listeriosis [8]. Levels below 100 CFU g−1 are generally accepted to be very low; however, most countries have a “zero-tolerance” policy regarding L. monocytogenes presence, due to potential risks [9]. Hence, the detection of the pathogen in food and environmental samples has emerged as a global necessity. The current detecting methods, however, have limitations due to their long procedural time (culture-based methods), high cost, need for highly trained staff (molecular-based methods), and low detection sensitivity (immunology-based methods) [10,11,12]. Therefore, new methods, such as biosensing techniques, have been at the epicenter of scientific interest, with numerous studies reporting high accuracy and sensitivity in an increasing field of applications (e.g., food quality and safety control, environmental monitoring, clinical diagnostics). Regarding the presence of L. monocytogenes in food, studies have reported the development of fiber-optic biosensors [13,14], piezoelectric cantilever biosensors [15], DNA or immuno-amperometric biosensors [16,17,18,19], and enzyme, DNA, or immuno-based impedance biosensors [20,21,22]. However, only a limited number of studies have been conducted utilizing cell-based biosensor for the detection of L. monocytogenes in food and beverage [23], even though live cell-based biosensor systems have been already used in environmental [24] and medical studies [25,26] with high success.
The aim of this study was to test a newly developed L. monocytogenes biosensor, previously reported by Hadjilouka et al. [27] on actual food samples and validate its ability to detect pathogens presence in different food substrates, identifying and eradicating possible impediments due to the matrix effect. The newly developed biosensor was proven to be a robust and selective tool for the detection of pathogens when applied in L. monocytogenes broth samples, with 88% accuracy, 2 log CFU mL−1 limit of detection, and no cross-reaction with Escherichia coli and other Listeria species. The system uses (i) a cell-based biosensor technology that measures the cell membrane potential changes according to the principle of the Bioelectric Recognition Assay (BERA), and (ii) a portable device developed by EMBIO Diagnostics called B.EL.D (Bio Electric Diagnostics). Furthermore, the system is able to connect via Bluetooth 4.0 with a smartphone, thus allowing the end-user to be instantly informed of the test result. Given that, in the last decade, the majority of listeriosis infection has been mainly associated with dairy products and fresh produce consumption, tests were performed in ready-to-eat lettuce salads, milk, and halloumi cheese samples. In addition, due to the fact that Listeria monocytogenes may be present in small numbers in food that are often accompanied by significantly larger numbers of other microorganisms, three different protocols were validated, based on the applied level of the selective enrichment (no enrichment, primary enrichment, primary and secondary enrichment protocols).
2. Materials and Methods
2.1. Materials and Reagents
Monkey African green kidney (Vero) cell cultures were provided by LGC Promochem (Teddington, UK). Fetal bovine serum (FBS), antibiotics (streptomycin–penicillin), L-glutamine & L-alanine, and trypsin/EDTA were purchased from Sigma-Aldrich (Taufkirchen, Germany). Phosphate Buffered Saline was obtained from MP Biomedicals (Illkrich, France). The anti-L. monocytogenes p60 protein antibody clone p6007 was purchased from antibodies-online.com. L. monocytogenes NCTC 11994 and sodium chloride were supplied by Merck (Darmstadt, Germany). Brain Heart Infusion was purchased from Biolife (Milan, Italy). Chromogenic Listeria agar, Palcam agar, Half-Fraser broth, and Full-Fraser broth, were obtained from Oxoid (Hampshire, UK).
2.2. Collection of Samples
Samples of ready-to-eat lettuce salads (n = 100) were purchased from supermarkets and grocery stores in Nicosia, Cyprus. Samples of milk (n = 100) and halloumi cheese (n = 100) were kindly provided by the Cypriot dairy industry Charalambides Christis. All the samples were transported to the laboratory in cool boxes to maintain the cooling chain and were analyzed the same day.
2.3. Cell Culture Conditions and Sensor Fabrication
Vero cells were cultured according to Apostolou et al. [28]. In brief, cells were cultured with a nutrient medium comprising of Dulbecco’s medium with 10% fetal bovine serum (FBS), 10% streptomycin/penicillin, and 10% L-glutamine and l-alanine and incubated at 5% CO2 and 37 °C.
Membrane-engineered cells were created by the electroinsertion of the anti-L. monocytogenes p60 protein antibody clone p6007 into the membrane of Vero cells, according to previously described protocols [27,28]. Briefly, the Vero cells were detached from the culture vessels using Trypsin/EDTA (10 min at 37 °C) and were collected by centrifugation (6 min/1000 rpm/25 °C), at a final density of 2.5 × 106 mL−1. The cell pellet was resuspended in 400 μL PBS containing 5 μg mL−1 antibody and incubated on ice for 20 min. Then, the cell–antibody mixture was transferred into electroporator (Eppendorf Eporator, Hamburg, Germany) cuvettes (4 mm), and electroinsertion was performed by the application of two square electric pulses of an electric field at 1800 V/cm. Subsequently, the mixture was transferred to a petri dish (60 × 15 mm) containing nutrient medium and overnight incubation took place at 37 °C and 5% CO2. The next day, the medium was discarded from the petri dish and the membrane-engineered cells were mechanically detached and collected with fresh nutrient medium in Eppendorf tubes.
2.4. Bacteria Culturing and Sample Inoculation
Prior to culture, Listeria monocytogenes NCTC 11994 was frozen stored at −20 °C in nutrient broth supplemented with 50% glycerol. Before experimental use, the pathogen was grown twice in Brain Heart Infusion broth at 37 °C for 24 h. Overnight L. monocytogenes culture (9 log CFU mL−1) was centrifuged (3500 rpm/ 10 min/ 4 °C), washed twice with sterile saline solution (NaCl 0.85%), resuspended in the same diluent, and serially diluted to inoculate food samples at 2, 4, and 6 log CFU mL−1 or g−1. Inoculation of the samples was performed as follows: 10 or 25 g or mL (depending on the protocol and on the food sample) of each food substrate was placed in sterile containers and sprayed with 500 μL of the appropriate pathogen dilution to achieve the desired final population. The subsequent sample treatment was performed according to the respective protocol (instant analysis or incubation prior to analysis).
2.5. Experimental Design (Protocols)
2.5.1. No Enrichment Protocol
No sample enrichment was applied in this protocol. Preparation of the tested samples and initial suspensions were performed according to ISO 6687-1:2017 [29] and ISO 11290-2:2017 [30] using sterile saline solution as a diluent. More specifically, 10 g or mL of the tested sample (inoculated or not) was transferred into a sterile stomacher bag and homogenized with 90 mL of sterile saline solution. After homogenization, a portion of the sample was used for analysis with the biosensor and a portion was used for serial dilutions and surface plating on Chromogenic Listeria agar and Palcam agar. In the case of the uninoculated samples, the presence of presumptive L. monocytogenes colonies was examined further through biochemical tests according to ISO 11290-2:2017, and through specific Polymerase Chain Reaction according to D’Agostino et al. [31].
One-hundred and fifty tests (150) were initially conducted on ready-to-eat lettuce salads, milk, and halloumi cheese samples (50 tests for each food category) (control samples). Control samples were tested to analyze the obtained signal from each food matrix without the presence of L. monocytogenes. To ensure the absence of pathogens, control samples were tested in parallel according to ISO 11290-2:2017. Subsequently, tests were conducted on 100 ready-to-eat lettuce salads, 100 milk and 100 halloumi samples artificially inoculated with L. monocytogenes at 3 different concentrations (2, 4, and 6 log CFU mL−1 or g−1).
2.5.2. Enrichment Protocols
In addition to the first protocol, two extra protocols were performed on samples treated with selective enrichments (either with primary enrichment or with primary and secondary enrichment). In these assays, preparation of the tested samples and initial suspensions were performed according to ISO 11290-1:2017 using Half-Fraser broth and Full-Fraser broth for primary and secondary enrichment, respectively. Half-Fraser broth was used as a diluent fluid in both protocols. More accurately, 25 g or 25 mL of the tested sample (inoculated or not) was transferred into a sterile stomacher bag and homogenized with 225 mL of Half-Fraser broth (primary selective enrichment). The initial suspension was incubated at 30 °C for 24 h. The next day, a portion of the incubated suspension was tested with the biosensor (primary enrichment protocol), while 0.1 mL of the suspension was inoculated into 10 mL of Full-Fraser broth (secondary selective enrichment) and incubated at 37 °C for 24 h. Tests were then conducted on the incubated samples (secondary enrichment protocol). In addition to the biosensor analysis, samples were also examined with the ISO 11290-1:2017 using the selective chromogenic media Chromogenic Listeria Agar and Palcam agar, for results validation. Furthermore, in the case of the uninoculated samples, the presence of presumptive L. monocytogenes colonies was examined further through biochemical tests according to ISO 11290-1:2017 and through specific PCR, as described in the first protocol (Section 2.5.1).
A total of 600 tests were conducted on control samples (100 tests for each food category × 2 protocols) to analyze the signal from each food matrix without the presence of the pathogenic bacterium. Six hundred tests (600) were then conducted on 100 ready-to-eat lettuce salads, 100 milk and 100 halloumi samples artificially inoculated with L. monocytogenes at 3 different concentrations (0.6, 2 and 4 CFU mL−1 or g−1) for both protocols.
2.6. Assay Procedure
2.6.1. Biosensor Device and Sample Loading
A customized hardware portable device developed by EMBIO DIAGNOSTICS (EMBIO DIAGNOSTICS Ltd., Cyprus) was used throughout this study. The device is a portable multichannel potentiometer with a replaceable connector of eight Screen-Printed Electrodes, which measures electric signals from biorecognition elements. Measurements are performed utilizing high-accuracy A/D converters, thus allowing multichannel, high-throughput, and rapid analyses. This tool is based on a powerful cell-based biosensor technology known as the Bioelectric Recognition Assay (BERA). Moreover, the system connects with a smartphone via Bluetooth 4.0, thus allowing the end-user to be instantly informed of the test result (Figure 1a).
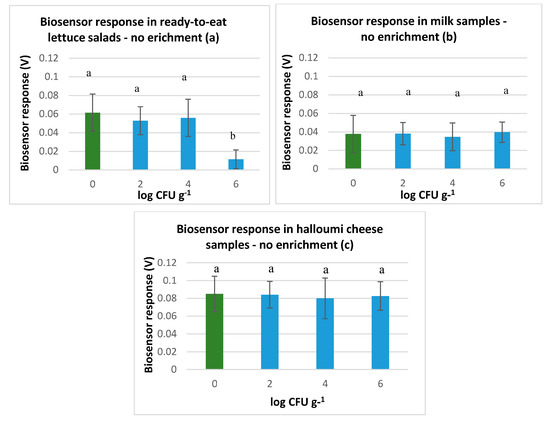
Figure 1.
Biosensor response in (a) ready-to-eat lettuce salad samples, (b) milk samples and (c) halloumi cheese samples, without L. monocytogenes (green color) and with L. monocytogenes (blue color) at 2.4 and 6 log CFU g−1, after homogenization with sterile saline solution (NaCl 0.85%). The error bars represent the standard errors of the mean value of all replications. The columns marked with different letters indicate that response was significantly (p < 0.05) different from the respective of the control samples (NaCl 0.85%) for each experimental assay.
A sample analysis was performed as previously described by Hadjilouka et al. [27]. In brief, 20 μL of each sample was added on the top of each gold screen-printed electrode and 20 μL of the membrane engineered cells (~5 × 104 cells) was added after 60 s. Each measurement lasted 3 min and 360 values were recorded at a sampling rate of 2 Hz for each sample. After each analysis, measurements were uploaded into a cloud server and based on a newly developed algorithm (Section 2.6.2), results were instantly calculated and appeared on the smartphone screen. Each sample was tested eight times by using a set of eight individual sensors and each experiment was performed in duplicate.
2.6.2. Algorithm for Signal Processing and Statistical Analysis
Each test produced a time-series comprising of 360 voltage detection measurements per sample. Response processing was performed according to Hadjilouka et al. [27]. In brief, a two-step analysis was conducted in python programming language using data analysis libraries, and four feature vectors were calculated based on (a) the rolling average with a rolling window size 50, and (b) the average values for each data set. These vectors were calculated for each electrode channel and in the overall test data set (8 electrodes). Consequently, eighteen feature values (8 values for each channel + 1 overall value/(a) and (b)) were used for the algorithm development and sample discrimination. These values obtained by the inoculated and uninoculated samples were compared and statistical differences were assessed through one-way analysis of variance (ANOVA). Subsequently, thresholds that classified samples were set to for each feature vector and method’s limit of detection (LOD) were determined for each food category. Data-stored result arrays were finally created for inoculated and uninoculated samples, thus allowing the system to classify instantly the samples above or Below the LOD, after each analysis.
Based on the comparison of the results obtained by the biosensor and the standard methods, performance indices were calculated for the evaluation of the newly developed method. More accurately, sensitivity (Se: the proportion of the positive samples that are correctly identified by the test), specificity (Sp: the proportion of the negative samples that are correctly identified by the test), positive predictive value (PPV: the probability that the sample is positive given a positive test result) and negative predictive value (NPV: the probability that the sample is negative given a negative test result) [32].
3. Results
3.1. No Enrichment Protocol
The results obtained from the tests conducted on ready-to-eat lettuce salads without enrichment indicate that the biosensor was able to discriminate samples with and without L. monocytogenes with high accuracy (92%) when pathogen population was 6 log CFU g−1. Furthermore, biosensors’ high sensitivity, specificity, positive predictive value, and negative predictive value (Se: 100%, Sp: 83%, PPV: 86%, and NPV: 100%) revealed considerable discriminating power. However, the biosensor was not able to distinguish sufficiently between positive and negative samples when L. monocytogenes was inoculated at 2 and 4 log CFU g−1. In that case, the method accuracy was 78%, and performance indices were sensitivity and positive predictive value of 83%, and specificity and negative predictive value of 67%. Similar results were observed for milk and halloumi samples when the pathogen was present at 2 or 4 log CFU mL−1 or g−1 (Figure 1a), indicating poor discrimination power. Specifically, biosensor accuracy was 56% and 67%, and its performance characteristics were Se: 50% and 91%, Sp: 59% and 40%, PPV: 45% and 63%, and NPV: 64% and 80% in milk and halloumi cheese samples, respectively. Nevertheless, contrarily to the biosensor’s ability to distinguish positive from negative ready-to-eat lettuce salads when the pathogen was at 6 log CFU g−1, the biosensor could not detect pathogen at this population level in milk and halloumi cheese samples (Figure 1b,c). Hence, it was indicated that with this protocol and sample treatment the biosensor was not able to discriminate samples with high accuracy unless samples were inoculated at the high population level of 6 log CFU g−1 and only in the case of ready-to-eat-lettuce salads.
3.2. Enrichment Protocols
The results obtained from the tests on ready-to-eat lettuce salads after the primary enrichment indicate that the biosensor was able to discriminate between samples with and without L. monocytogenes with very high accuracy (98%), at every studied concentration of the pathogen. Incubation with the primary enrichment medium augmented (5 log CFU g−1 or mL−1) the pathogen’s population at high levels, increasing biosensor’s ability to distinguish positive from negative samples, even when the pathogen was initially inoculated at low levels (0.6 log CFU g−1 or mL−1) (Figure 2a). Accordingly, the biosensor gave similar results when testing milk and halloumi cheese samples after the primary enrichment (Figure 2b,c). The biosensor’s accuracy was 90 and 91% in milk and halloumi samples, respectively and performance indices were 90% sensitivity, 91% specificity, 93% positive predictive value, and 89% negative predictive value in milk, and 91% sensitivity, 91% specificity, 95% positive predictive value, and 86% negative predictive value in halloumi cheese. Furthermore, the biosensor revealed infallible discrimination power (100% accuracy, sensitivity, specificity, PPV and NPV) when the limit of detection was set at 2 log CFU mL−1 or g−1. The results indicate that the potential dynamic of the samples was decreasing almost to a linear pattern against increasing L. monocytogenes concentrations.

Figure 2.
Biosensor response in (a) ready-to-eat lettuce salad samples, (b) milk samples and (c) halloumi cheese samples without L. monocytogenes (green color) and with L. monocytogenes (blue color) at 0.6, 2 and 4 log CFU g−1 (initial inoculation level) after incubation with primary enrichment broth (Half Fraser). The error bars represent the standard errors of the mean value of all replications. The columns marked with different letters indicate that response was significantly (p < 0.05) different from the respective of the control samples (samples without L. monocytogenes incubated with the primary enrichment) for each experimental assay.
Contrarily to these results, tests on samples that were initially incubated with the primary enrichment and then with the secondary enrichment did not manage to produce trustworthy results regarding the pathogen’s absence/presence. More accurately, the biosensor was able to discriminate negative from positive samples only when L. monocytogenes initial inoculation level was at 4 log CFU g−1 or mL−1. However, this discrimination was not statistically significant (p < 0.05). This was observed for all the food substrates (Figure 3).
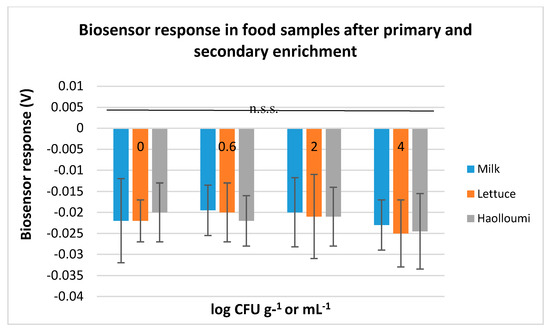
Figure 3.
Biosensor response in milk, ready-to-eat lettuce salad, and halloumi cheese without L. monocytogenes (0 log CFU g−1 or mL−1) and with L. monocytogenes (0.6, 2, and 4 log CFU g−1 or mL−1 initial inoculation level) after incubation with the primary enrichment broth (Half Fraser) and then with the secondary enrichment broth (Full Fraser). The error bars represent the standard errors of the mean value of all replications. n.s.s: non-statistically significant different results (p < 0.05).
Since samples were added on the electrodes and the biosensors were added after 60 s, the potential dynamic of the samples with and without Listeria monocytogenes prior to biosensor’s addition was also examined (Figure 4). The results indicate that the potential dynamic of the inoculated samples (voltage measurements from 0 to 120) was decreasing against the increasing L. monocytogenes concentrations (Figure 5).

Figure 4.
Time series of the 360 voltage detection measurements obtained from four different electrodes testing milk samples with four different L. monocytogenes concentrations (0, 0.6, 2, and 4 log CFU mL−1) after incubation with the primary enrichment broth. Samples were added on each electrode and measurements were recorded for 60 s. The biosensors were then added (red box) and the measurements recorded describe the reaction between the sample and the biosensor (60–180 s/120–360 measurements).
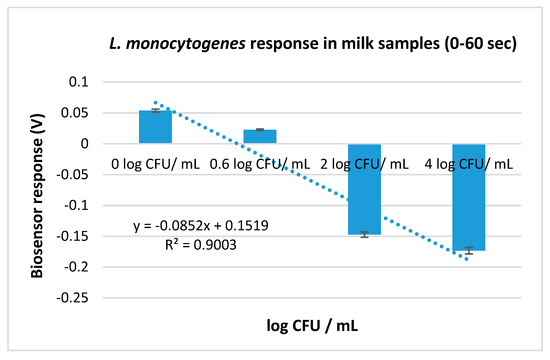
Figure 5.
L. monocytogenes response in milk samples with four different initial concentrations (0, 0.6, 2, and 4 log CFU mL−1) after incubation with the primary enrichment broth. The error bars represent the standard errors of the mean value of all replications. The columns are marked with different letters, indicating that response was significantly (p < 0.05) different from the respective of the control samples (samples incubated with the primary enrichment without L. monocytogenes (0 log CFU mL−1)) for each experimental assay.
3.3. Database Creation
Afterward, and while the detection method was demonstrated, as shown above (Section 3.2), to present high accuracy when the detection limit was set to 0.6 log CFU mL−1 or g−1, a database was created in order to give an immediate and automated result to the user without the need for any further processing. The results used to create the database were previously processed according to the algorithm developed and described in Section 2.6.2. The available data were 1600 time-series (200 tests (100 inoculated and 100 uninoculated) × 8 electrodes), each containing 360 measurements. Since the LOD of the method was set at 0.6 log CFU mL−1 or g−1, any test with a concentration equal to or greater than 0.6 log CFU mL−1 or g−1 was considered as ‘Above LOD’ and any test with a lower concentration (control samples) was considered as ‘Below LOD’. Specifically, 800 time-series were included with the ‘Above LOD samples’ and 800 time series with the ‘Below LOD samples’.
The results obtained from the use of the biosensor in different samples reveal the ability of the system to be used as a screening method for the rapid detection of the pathogenic bacterium in these types of food and its classification into ‘Above or Below’ categories. Based on the data extracted from the experiments, a user-friendly interface was created, which, after comparing each test with the ‘Above’ and ‘Below’ database, can produce a user-readable result.
The Backend platform that stores the information in real-time, is the Google Firestore [33] and the algorithm was written in Python and Javascript and uploaded in Google Cloud Functions. The data were stored in Google Storage on a Horizontal database.
4. Discussion
Listeria monocytogenes is a pathogen that causes listeriosis, a relatively rare foodborne disease with a high, nevertheless, mortality rate. Pathogen’s ubiquitous nature in combination with its peculiarity to grow at refrigeration temperatures, and its ability to survive in adverse conditions [34] and form biofilms [35] explain why L. monocytogenes is one of the most significant concerns in the food industry. This concern is particularly important for ready-to-eat foods that have not been subjected to any form of thermal processing or any other antimicrobial step before consumption.
In the United States, Australia, and New Zealand, regulations require the absence of L. monocytogenes through products’ shelf life [36]. However, the regulations in Europe (EC No 2073/2005) and Canada allow up to 100 CFU g−1 in ready-to-eat foods, provided that they do not support the growth of the pathogen. ‘Zero tolerance’ is required for ready-to-eat foods intended for special medical purposes and infants, and for ready-to-eat foods that support the growth of L. monocytogenes, such as milk and dairy products. For the latter case, the European regulation places the responsibility on the food business operators to investigate products’ compliance with the criteria through their shelf life. However, despite the industry’s efforts to control L. monocytogenes, the pathogen cannot be eliminated and listeriosis outbreaks keep occurring. Hence, a decrease of pathogen’s prevalence and control of its presence in the food processing industry are of great importance to public health. The aim of this study was to test a newly developed L. monocytogenes biosensor, previously reported by our team, on actual food samples and validate its discrimination ability between negative and positive samples. The newly developed biosensor system utilizes a cell-based biosensor technology and a portable device, and it is able to provide results within 3 min. In the last decade, the majority of the listeriosis outbreaks have been mainly connected to fresh produce and dairy product consumption, tests were conducted on ready-to-eat lettuce salads, milk, and halloumi cheese samples, and three different protocols were conducted for each food substrate based on the number of the selective enrichment broths applied.
This study’s results indicate that the ‘no-enrichment’ protocol was not able to discriminate positive from negative food samples with high accuracy unless the pathogen was present at a high population level in ready-to-eat salads (LOD: 6 log CFU g−1). This, however, was not observed in our previous study, in which the same biosensor was successfully used for the detection of L. monocytogenes in broth samples. More accurately, in that study, the pathogen was inoculated in sterile saline solution at four different population levels (2, 4, 6, and 9 log CFU mL−1) and tests were conducted on the inoculated broths [27]. The results indicated that the biosensor was able to detect the pathogenic bacterium with high accuracy and LOD 2 log CFU mL−1 in these broth samples. Hence, the biosensor’s lack of detection ability that was observed in the ‘no-enrichment’ protocol of this study, in which food samples were inoculated with L. monocytogenes at the respective population levels (2, 4, and 6 log CFU mL−1 or g−1) and homogenized with sterile saline solution, was attributed to impediments due to the food matrix effect. These impediments were more significant in the case of the milk and halloumi, since the biosensor was not able to detect the pathogen even at 6 log CFU ml−1 or g−1, probable due to dairy products’ complex constituents. Milk is considered nature’s most complete food, consisting of fat, proteins, salts, enzymes, and vitamins, with major physiological and biochemical functions [37]. At the same time, dairy products are considered as the most nutritious foods. Halloumi cheese, the traditional cheese of Cyprus, mainly consists of fat, proteins, and sodium chloride [38]. On the other hand, lettuce mainly consists of water (95%) and contains small amounts of fiber and minerals [39], while fresh produce, with few exceptions, are defined as ‘sodium free’ since they have a negligible amount of salt. In cheese, however, salt levels significantly contribute to products’ flavor and quality [40] as well as antimicrobial environment. Similarly, milk contains large amounts of salts (phosphates, citrates, potassium, bicarbonates of sodium, etc.), while its proteins can form salts with counter-ions, due to their positively and negatively charged groups [41]. Thus, it was concluded that milk and cheese matrices affected the measured potential dynamic in a more significant way than fresh produce matrix did, due to their constituents that overlapped the potential dynamic changes occurring due to a pathogen’s presence. Moreover, the matrix seemed to negatively affect the method’s ability to detect the pathogen in milk and halloumi cheese samples when no-enrichment protocol was conducted, due to the pathogen’s location within the dairy products. Bacteria (starter, non-starter, spoilage and pathogenic) are not homogenously distributed throughout dairy products, but they have been demonstrated to favorably establish at the fat–protein interface and occasionally being entrapped within the whey pockets [42]. This distribution makes it difficult for the rapid methods that have no enrichment step—like the first studied protocol—to detect a pathogens’ presence with high accuracy in such food substrates.
Therefore, two extra protocols that included enrichment steps were also studied. In food, L. monocytogenes may be present in small concentrations that are often accompanied by considerably larger numbers of other microorganisms. Furthermore, even though the pathogen is resistant in adverse conditions, it can be seriously stressed by the processing treatments [43,44]. Detecting L. monocytogenes in foodstuff even if it is present in small numbers or in stressed and injured states is crucial, since small numbers can grow into large populations and injured or stressed cells can regain their viability and pathogenicity under suitable conditions [45]. To eliminate this risk, the enrichment of the tested samples is recommended ([10], ISO detection), even for gene-based [46] or immunologically based [47] procedures that can detect a pathogen’s presence within considerably shorter time periods in comparison to the standard methods. For this reason, the ’primary-enrichment’ and ‘secondary-enrichment’ protocols were also examined, involving one or two enrichment steps, respectively.
The results obtained from the ‘secondary-enrichment’ protocol indicate the biosensor’s inability to discriminate positive from negative samples, despite the pathogen’s high population levels when present. This inability, however, was attributed to the matrix effect of the secondary enrichment medium and not to the matrix effect of the food substrates since biosensor’s potentiometric responses were similar with no statistically significant differences between all the food categories. Contrarily to these results, the ‘primary-enrichment’ protocol revealed the biosensor’s high discrimination power.
Furthermore, the method’s LOD was determined to be as low as 0.6 log CFU mL−1 or g−1 in all food categories. Hence, it was decided that the ‘primary-enrichment’ protocol that requires enrichment of the samples with Half Fraser broth at 30 °C for 24 h was the most appropriate for the newly developed biosensor system.
Finally, it is noteworthy that from the ‘primary-enrichment’ protocol, it was noticed that the potential dynamic of the samples was decreasing against the increasing L. monocytogenes concentrations, almost to a linear pattern. The electrochemical potential is the released energy that comes of the translocation of the ions across the membrane. This translocation is considerably important for the physiology of the cells. Nevertheless, bacterial electrophysiology is an unexplored field that did not draw the attention of microbiologists for many years. This lack of interest was due to the established knowledge that the electrical potential is of great importance in fundamental cellular functions (e.g., ATP synthesis, membrane transport) and to the general assumption that it was homeostatic. This, however, has been recently revised, since it has been revealed that the bacterial membrane potential is dynamic and substantial in microbes’ behavior (e.g., intercellular communication, environmental sensing) [48,49]. It was therefore concluded that the potential dynamic was differentiating among samples not only due to the membrane potential changes resulting from the antigen-electroinserted antibody binding, but also due to the membrane potential changes that take place in the bacteria cells. However, since electrophysiology is mostly an unexplored field, further research is needed. Despite the considerable differences observed in the ‘primary-enrichment’ protocol between inoculated and uninoculated samples before the biosensor’s addition, the method was not able to distinguish positive from negative samples with high accuracy based only on these differences, unless the biosensor was added. The data obtained after the biosensor’s addition were important for the algorithm development, thus indicating that the reaction between the antigen and the antibody is the method’s integral part.
5. Conclusions
The present study demonstrates a portable cell-based biosensor system that is able to detect L. monocytogenes in ready-to-eat lettuce salads, milk and halloumi cheese. The method is based on the ISO 11290-1 standard, facilitating its integration in laboratories routine diagnostics. It requires a 24 h enrichment step and provides results within 3 min. Furthermore, it is combined with an algorithm embedded in a user-friendly software that connects via Bluetooth 4.0 with an android device, thus allowing the end-user to be instantly informed of the test result. The newly developed method allows the fast and sensitive detection of L. monocytogenes in ready-to-eat lettuce salads, milk and halloumi cheese, with 98%, 90%, and 91% accuracy, respectively, and with a limit of detection of 0.6 log CFU g−1 or mL−1 in all the food substrates. Thus, it could be used as a screening method for the rapid detection of the pathogenic bacterium in these food types.
Author Contributions
Conceptualization, D.T. and K.L.; methodology, A.H.; software, L.D.; A.I.; data curation, A.H., T.A., L.D., A.I. and K.L.; validation, A.H. and T.A.; writing—original draft preparation, A.H. and T.A.; writing—review and editing, D.T.; Visualization, K.L.; supervision, D.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The RESTART 2016–2020 Programmes for Research, Technological Development and Innovation of the Research and Innovation Foundation (RIF) in Cyprus. (Project “Post-Doc/0718/0003”—Post Doctoral Researchers Programme).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). Listeriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 19 August 2020).
- CDC (Center of Disease Control and Prevention). Listeria (Listeriosis). Listeria Outbreaks. Available online: https://www.cdc.gov/listeria/outbreaks/index.html (accessed on 20 August 2020).
- Pearson, J.L.; Marth, H.E. Listeria monocytogenes—Threat to a safe food supply: A review 1. J Dairy Sci. 1990, 73, 912–928. [Google Scholar] [CrossRef]
- Jayamanne, S.V.; Samarajeewa, U. Evaluation of the resistance of pathogenic Listeria monocytogenes in milk and milk products in Sri Lanka. Trop. Agric. Res. Ext. 2010, 13, 73–80. [Google Scholar] [CrossRef][Green Version]
- Kasalica, A.; Vuković, V.; Vranješ, A.; Memiši, N. Listeria monocytogenes in milk and dairy products. Biotechnol. Anim. Husb. 2011, 27, 1067–1082. [Google Scholar] [CrossRef]
- Hadjilouka, A.; Paramithiotis, S.; Drosinos, E.H. Prevalence of Listeria monocytogenes and occurrence of listeriosis from ready to eat fresh fruits and vegetables. In Listeria monocytogenes: Food Sources, Prevalence and Management Strategies; Hambrick, E.C., Ed.; Nova Publishers: New York, NY, USA, 2014; pp. 283–296. [Google Scholar]
- Shelef, L.A. Listeriosis and transmission by food. Proces. Food Nutri. Sci. 1989, 13, 363–382. [Google Scholar]
- Ooi, S.T.; Lorber, B. Gastroenteritis due to Listeria monocytogenes. Clin. Infect. Dis. 2005, 40, 1327–1332. [Google Scholar] [CrossRef]
- Farber, J.M.; Zwietering, M.; Wiedmann, M.; Schaffner, D.; Hedberg, C.W.; Harrison, M.A.; Hartnett, E.; Chapman, B.; Donnelly, C.W.; Goodburn, K.E.; et al. Alternative approaches to the risk management of Listeria monocytogenes in low risk foods. Food Control 2020, 107601. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method; Reference number: IS0 11290-1:2017; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Ali, S.; Hassan, A.; Hassan, G.; Eun, C.; Bae, J.; Lee, C.H.; Kim, I. Disposable all-printed electronic biosensor for instantaneous detection and classification of pathogens. Sci. Rep. 2018, 8, 5920. [Google Scholar] [CrossRef]
- Kanayeva, D.A.; Wang, R.; Rhoads, D.; Erf, G.F.; Slavik, M.F.; Tung, S.; Li, Y. Efficient separation and sensitive detection of Listeria monocytogenes using an impedance immunosensor based on magnetic nanoparticles, a microfluidic chip, and an interdigitated microelectrode. J. Food Prot. 2012, 75, 1951–1959. [Google Scholar] [CrossRef]
- Geng, T.; Morgan, M.T.; Bhunia, A.K. Detection of low levels of Listeria monocytogenes cells by using a fiber-optic immunosensor. Appl. Environ. Microbiol. 2004, 70, 6138–6146. [Google Scholar] [CrossRef]
- Ohk, S.H.; Koo, O.K.; Sen, T.; Yamamoto, C.M.; Bhunia, A.K. Antibody-aptamer functionalized fibre-optic biosensor for specific detection of Listeria monocytogenes from food. J. Appl. Microbiol. 2010, 109, 808–817. [Google Scholar] [CrossRef]
- Sharma, H.; Mutharasan, R. Rapid and sensitive immunodetection of Listeria monocytogenes in milk using a novel piezoelectric cantilever sensor. Biosens. Bioelectron. 2013, 45, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Liu, Q.J.; Wu, Z.W.; Lu, Z.H. Electrochemical detection of toxin gene in Listeria monocytogenes. Hereditas 2010, 32, 512–516. [Google Scholar] [PubMed]
- Sun, W.; Qi, X.; Zhang, Y.; Yang, H.; Gao, H.; Chen, Y.; Sun, Z. Electrochemical DNA biosensor for the detection of Listeria monocytogenes with dendritic nanogold and electrochemical reduced graphene modified carbon ionic liquid electrode. Electrochim. Acta. 2012, 85, 145–151. [Google Scholar] [CrossRef]
- Davis, D.; Guo, X.; Musavi, L.; Lin, C.S.; Chen, S.H.; Wu, V.C.H. Gold nanoparticle-modified carbon electrode biosensor for the detection of Listeria monocytogenes. Ind. Biotechnol. 2013, 9, 31–36. [Google Scholar] [CrossRef]
- Cheng, C.; Peng, Y.; Bai, J.; Zhang, X.; Liu, Y.; Fan, X.; Ning, B.; Gao, Z. Rapid detection of Listeria monocytogenes in milk by self-assembled electrochemical immunosensor. Sens. Actuators B Chem. 2014, 190, 900–906. [Google Scholar] [CrossRef]
- Tolba, M.; Ahmed, M.U.; Tlili, C.; Eichenseher, F.; Loessner, M.J.; Zourob, M. A bacteriophage endolysin-based electrochemical impedance biosensor for the rapid detection of Listeria cells. Analyst 2012, 137, 5749–5756. [Google Scholar] [CrossRef]
- Soni, D.K.; Prakash, R.; Dubey, S.K. Label-free impedimetric detection of Listeria monocytogenes based on poly-5-carboxy indole modified ssDNA probe. J. Biotechnol. 2015, 200, 70–76. [Google Scholar]
- Wang, R.; Dong, W.; Ruan, C.; Kanayeva, D.; Tian, R.; Lassiter, K.; Li, Y. TiO2 nanowire bundle microelectrode-based impedance immunosensor for rapid and sensitive detection of Listeria monocytogenes. Nano Lett. 2008, 8, 2625–2631. [Google Scholar] [CrossRef]
- Banerjee, P.; Lenz, D.; Robinson, J.P.; Rickus, J.L.; Bhunia, A.K. A novel and simple cell-based detection system with a collagen-encapsulated B-lymphocyte cell line as a biosensor for rapid detection of pathogens and toxins. Lab. Investig. 2008, 88, 196–206. [Google Scholar] [CrossRef]
- Perdikaris, A.; Vassilakos, N.; Yiakoumettis, I.; Kektsidou, O.; Kintzios, S. Development of a portable, high throughput biosensor system for rapid plant virus detection. J. Virol. Methods 2011, 177, 94–99. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Tsekouras, V.; Kintzios, S. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein antigen. Biosensors 2020, 20, 3121. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Vitsa, K.; Bem, F.; Vassilakos, N.; Perdikaris, A.; Blouhos, P.; Yialouris, C.; Frossiniotis, D.; Anthopoulos, I.; Maggana, O.; et al. Engineering of the membrane of fibroblast cells with virus-specific antibodies: A novel biosensor tool for virus detection. Biosens. Bioelectron. 2008, 24, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Hadjilouka, A.; Loizou, K.; Apostolou, T.; Dougiakis, L.; Inglezakis, A.; Tsaltas, D. Newly developed system for the robust detection of Listeria monocytogenes based on a bioelectric cell biosensor. Biosensors 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, T.; Loizou, K.; Hadjilouka, A.; Inglezakis, A.; Kintzios, S. Newly developed system for acetamiprid residue screening in the lettuce samples based on a bioelectric biosensor. Biosensors 2020, 10, 8. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Microbiology of the Food Chain—Preparation of the Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions: ISO 6887-1:2017; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 2: Enumeration Method; Reference number: IS0 11290-2:2017; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- D’Agostino, M.; Wagner, M.; Vazquez-Boland, J.A.; Kuchta, T.; Karpiskova, R.; Hoorfar, J.; Novella, S.; Scortti, M.; Ellison, J.; Murray, A.; et al. A validated PCR-based method to detect Listeria monocytogenes using raw milk as a food model—Towards an international standard. J. Food Prot. 2004, 67, 1646–1655. [Google Scholar] [CrossRef]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef]
- van der Walt, S.; Colbert, S.C.; Varoquaux, G. The NumPy Array: A Structure for Efficient Numerical Computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.; Deng, L.; Feng, F.; Wang, L.; Zhou, Q.; Luo, Q. SigB plays a major role in Listeria monocytogenes tolerance to bile stress. Int. J. Food Microbiol. 2011, 145, 238–243. [Google Scholar] [CrossRef]
- Cruz, C.D.; Fletcher, G.C. Prevalence and biofilm-forming ability of Listeria monocytogenes in New Zealand mussel (Perna canaliculus) processing plants. Food Microbiol. 2011, 28, 1387–1393. [Google Scholar] [CrossRef]
- Donoso, W.; Castro, R.I.; Guzmán, L.; López-Cabaña, Z.; Nachtigall, F.M.; Santos, L.S. Fast detection of Listeria monocytogenes through a nanohybrid quantum dot complex. Anal. Bioanal. Chem. 2017, 409, 5359–5371. [Google Scholar] [CrossRef]
- Mehta, B.M. Chemical Composition of Milk and Milk Products. In Handbook of Food Chemistry; Cheung Chi-Keung, P., Mehta, B.M., Eds.; Springer: Berlin, Germany, 2015; pp. 1–34. [Google Scholar]
- Papademas, P.; Robinson, R.K. Halloumi cheese: The product and its characteristics. Int. J. Dairy Technol. 2007, 51, 98–103. [Google Scholar] [CrossRef]
- Manukovsky, N.S.; Kovalev, V.S.; Tikhomirov, A.A.; Kalacheva, G.S.; Kolmakova, A.A. The Giant African Land Snail Achatina fulica (Bowdich, 1720) as a Candidate Species for Bioregenerative Life Support Systems. 2015. J. Sib. Fed. Univ. Biol. 2015, 8, 18–31. [Google Scholar] [CrossRef]
- Guinee, T.P. Salts in cheese. In Cheese Problems Solved; McSweeney, P.L., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; CRC Press: Cambridge, UK, 2007; pp. 80–89. [Google Scholar]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Salts of Milk. In Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 241–270. [Google Scholar]
- Hauke, A.; Oertel, S.; Knoke, L.; Fein, V.; Maier, C.; Brinkmann, F.; Jank, M.P. Screen-Printed Sensor for Low-Cost Chloride Analysis in Sweat for Rapid Diagnosis and Monitoring of Cystic Fibrosis. Biosensors 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; Daley, E.; Coates, F.; Emmons, D.B.; McKellar, R. Factors influencing survival of Listeria monocytogenes in milk in a high temperature short-time pasteurizer. J. Food Prot. 1992, 55, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.; Smith, L.T.; Smith, G.M. Glycine betaine confers enhanced osmotolerance and cryotolerance in Listeria monocytogenes. J. Bacteriol. 1994, 176, 426–431. [Google Scholar] [CrossRef]
- McCarthy, S.A. Pathogenicity of nonstressed, heat-stressed, and resuscitated Listeria monocytogenes. Appl. Environ. Microbiol. 1991, 57, 2389–2391. [Google Scholar] [CrossRef]
- Dalmasso, M.; Bolocan, A.S.; Hernandez, M.; Kapetanakou, A.E.; Kuchta, T.; Manios, S.G.; Melero, B.; Minarovičová, J.; Muhterem, M.; Nicolau, A.I.; et al. Comparison of polymerase chain reaction methods and plating for analysis of enriched cultures of Listeria monocytogenes when using the ISO11290-1 method. J. Microbiol. Methods 2014, 98, 8–14. [Google Scholar] [CrossRef]
- Gasanov, U.; Hughes, D.; Hansbro, P.M. Methods for the isolation and identification of Listeria spp. and Listeria monocytogenes: A review. FEMS Microbiol. Rev. 2005, 29, 851–875. [Google Scholar] [CrossRef]
- Benarroch, J.M.; Asally, M. The microbiologist’s guide to membrane potential dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef]
- Dai, J.; Ting-Beall, H.P.; Sheetz, M.P. The secretion-coupled endocytosis correlates with membrane tension changes in RBL 2H3 cells. J. Gen. Physiol. 1997, 110, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).