Developing Technologies for Biological Experiments in Deep Space †
Abstract
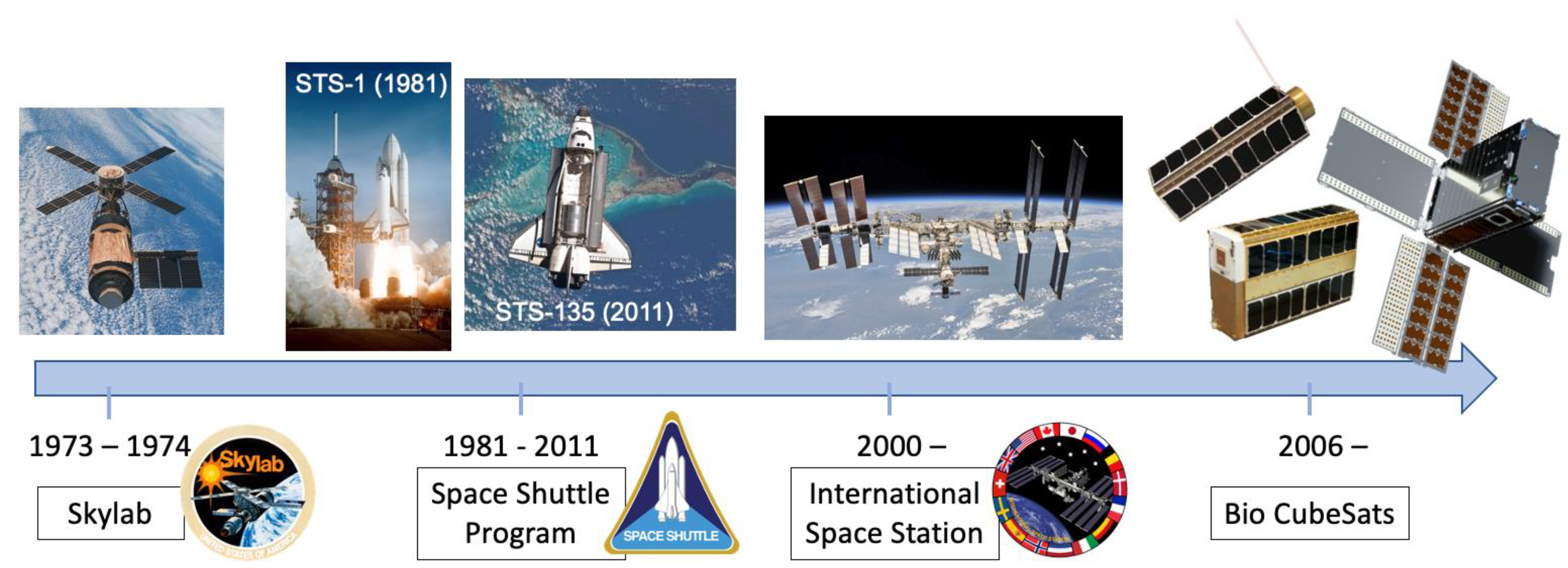
:1. Introduction
2. Past and Current Technologies
3. Biological CubeSat Missions
4. Future Technologies and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bizzarri, M.; Monici, M.; van Loon, J.J.W.A. How Microgravity Affects the Biology of Living Systems. BiomMed Res. Int. 2015, 2015, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ferl, R.J.; Paul, A.-L. The effect of spaceflight on the gravity-sensing auxin gradient of roots: GFP reporter gene microscopy on orbit. NPJ Microgravity 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W. Impact of space flight on bacterial virulence and antibiotic susceptibility. Infect. Drug Resist. 2015, 8, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space Radiation Biology for “Living in Space”. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Padgen, M.R.; Chinn, T.N.; Friedericks, C.R.; Lera, M.P.; Chin, M.; Parra, M.P.; Piccini, M.E.; Ricco, A.J.; Spremo, S.M. The EcAMSat fluidic system to study antibiotic resistance in low earth orbit: Development and lessons learned from space flight. Acta Astronaut. 2020, 173, 449–459. [Google Scholar] [CrossRef]
- Diaz-Aguado, M.F.; Ghassemieh, S.; Van Outryve, C.; Beasley, C.; Schooley, A. Small Class-D spacecraft thermal design, test and analysis—PharmaSat biological experiment. In Proceedings of the 2009 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2009; pp. 1–9. [Google Scholar] [CrossRef]
- NASA Technical Reports Server (NTRS). Available online: https://ntrs.nasa.gov/citations/19780017172 (accessed on 14 October 2020).
- NASA Technical Reports Server (NTRS). Available online: https://ntrs.nasa.gov/citations/19740025164 (accessed on 14 October 2020).
- NASA Technical Reports Server (NTRS). Available online: https://ntrs.nasa.gov/citations/19980206462 (accessed on 14 October 2020).
- Mobile SpaceLab. Available online: https://www.nasa.gov/mission_pages/station/research/experiments/explorer/Facility.html?#id=7692 (accessed on 14 October 2020).
- BioChip SpaceLab. Available online: https://www.nasa.gov/mission_pages/station/research/experiments/explorer/Facility.html?#id=7666 (accessed on 14 October 2020).
- CellCult. Available online: https://www.nasa.gov/mission_pages/station/research/experiments/explorer/Facility.html?#id=377 (accessed on 14 October 2020).
- Ricco, A.J.; Hines, J.W.; Piccini, M.; Parra, M.; Timucin, L.; Barker, V.; Storment, C.; Friedericks, C.; Agasid, E.; Beasley, C.; et al. Autonomous genetic analysis system to study space effects on microorganisms: Results from orbit. In Proceedings of the TRANSDUCERS 2007–2007 International Solid-State Sensors, Actuators and Microsystems Conference, Lyon, France, 10–14 June 2007; pp. 33–37. [Google Scholar] [CrossRef]
- Ricco, A.J.; Parra, M.; Niesel, D.; Piccini, M.; Ly, D.; McGinnis, M.; Kudlicki, A.; Hines, J.W.; Timucin, L.; Beasley, C.; et al. PharmaSat: Drug dose response in microgravity from a free-flying integrated biofluidic/optical culture-and-analysis satellite. In Proceedings of the SPIE 7929, Microfluidics, BioMEMS, and Medical Microsystems IX, San Francisco, CA, USA, 14 February 2011. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Ricco, A.J.; Agasid, E.; Beasley, C.; Diaz-Aguado, M.; Ehrenfreund, P.; Friedericks, C.; Ghassemieh, S.; Henschke, M.; Hines, J.W.; et al. The O/OREOS mission: First science data from the Space Environment Survivability of Living Organisms (SESLO) payload. Astrobiology 2011, 11, 951–958. [Google Scholar] [CrossRef]
- Salim, W.W.A.W.; Park, J.; Rickus, J.L.; Rademacher, A.; Ricco, A.J.; Schooley, A.; Benton, J.; Wickizer, B.; Martinez, A.; Mai, N.; et al. SporeSat: A nanosatellite platform lab-on-a-chip system for investigating gravity threshold of fern-spore single-cell calcium ion currents. In Proceedings of the Solid-State Sensors, Actuators and Microsystems Workshop, Hilton Head Island, SC, USA, 8–12 June 2014; pp. 111–114. [Google Scholar] [CrossRef]
- Massaro Tieze, S.; Liddell, L.C.; Santa Maria, S.R.; Bhattacharya, S. BioSentinel: A Biological CubeSat for Deep Space Exploration. Astrobiology 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Ricco, A.J.; Santa Maria, S.R.; Hanel, R.P.; Bhattacharya, S. BioSentinel: A 6U Nanosatellite for Deep-Space Biological Science. IEEE Aerosp. Electron. Syst. Mag. 2020, 35, 6–18. [Google Scholar] [CrossRef]
- Santa Maria, S.R.; Marina, D.B.; Massaro Tieze, S.; Liddell, L.C.; Bhattacharya, S. BioSentinel: Long-Term Saccharomyces cerevisiae Preservation for a Deep Space Biosensor Mission. Astrobiology 2020, 20. [Google Scholar] [CrossRef]
| CubeSat Mission (Size; Launch) | Biological Study | Research Investigation | Technology Development |
|---|---|---|---|
| GeneSat-1 (3U; 2006) | E. coli (bacterium) | Microgravity effects on gene expression |
|
| PharmaSat (3U; 2009) | S. cerevisiae (yeast) | Microgravity effects on antifungal response |
|
| O/OREOS SESLO (3U; 2010) | B. subtilis (bacterium) | Microgravity & LEO radiation effects |
|
| SporeSat (3U; 2014) | C. richardii (fern spores) | Microgravity effects on calcium transport |
|
| EcAMSat (6U; 2017) | E. coli (uropathogenic) | Microgravity effects on antibiotic response |
|
| BioSentinel (6U; 2021/2022) | S. cerevisiae | Deep space radiation effects |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawkins, E.M.; Kanapskyte, A.; Maria, S.R.S. Developing Technologies for Biological Experiments in Deep Space. Proceedings 2020, 60, 28. https://doi.org/10.3390/IECB2020-07085
Hawkins EM, Kanapskyte A, Maria SRS. Developing Technologies for Biological Experiments in Deep Space. Proceedings. 2020; 60(1):28. https://doi.org/10.3390/IECB2020-07085
Chicago/Turabian StyleHawkins, Elizabeth M., Ada Kanapskyte, and Sergio R. Santa Maria. 2020. "Developing Technologies for Biological Experiments in Deep Space" Proceedings 60, no. 1: 28. https://doi.org/10.3390/IECB2020-07085
APA StyleHawkins, E. M., Kanapskyte, A., & Maria, S. R. S. (2020). Developing Technologies for Biological Experiments in Deep Space. Proceedings, 60(1), 28. https://doi.org/10.3390/IECB2020-07085





