Fabrication of an All-Solid-State Ammonium Ion–Selective Electrode by a Two-Step Process Using Cyclic Voltammetry †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
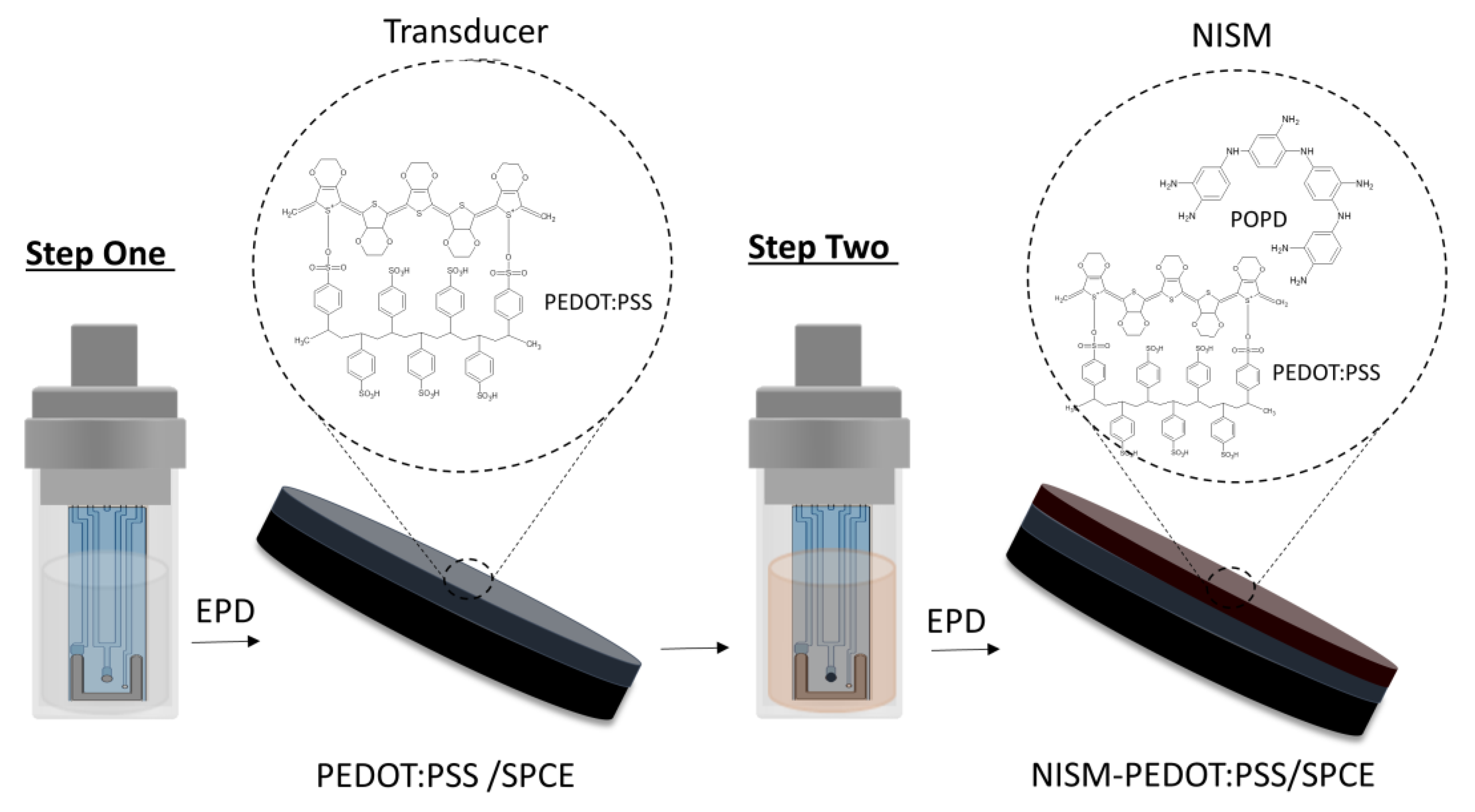
2.2. Fabrication of All-Solid-State Ammonium Ion–Selective Electrode (AS-NISE)
2.3. Characterization of PEDOT:PSS Transducer
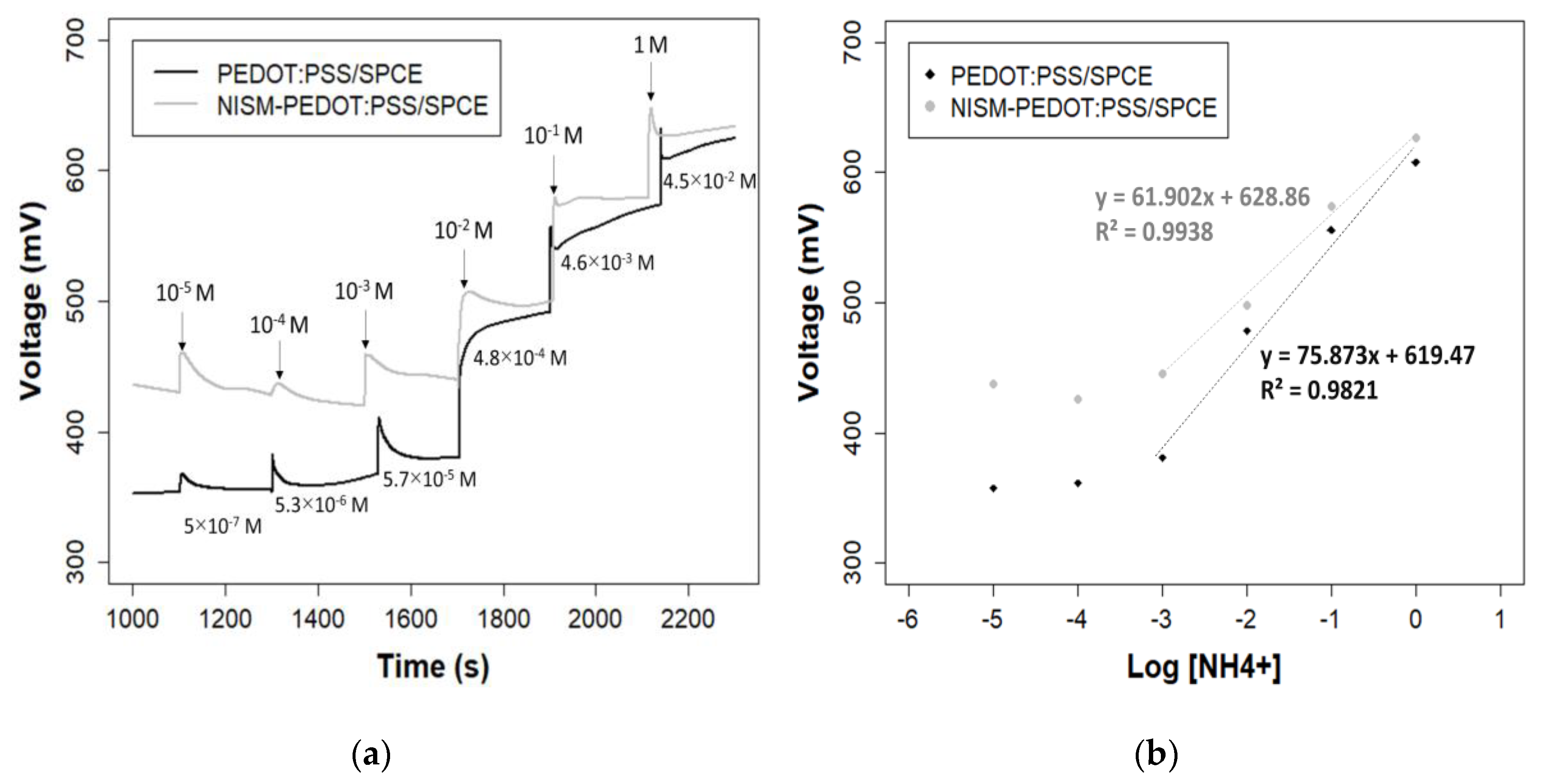
2.4. Potentiometry Measurement
3. Results
3.1. PEDOT:PSS/SPCE Transducer
3.2. Calibration Curve
4. Conclusions
Acknowledgments
References
- Delwiche, C.C. The nitrogen cycle. Sci. Am. 1970, 223, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Athavale, R.; Kokorite, I.; Dinkel, C.; Bakker, E.; Wehrli, B.; Crespo, G.A.; Brand, A. In situ ammonium profiling using solid-contact ion-selective electrodes in eutrophic lakes. Anal. Chem. 2015, 87, 11990–11997. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Kan, Y.T.; Han, C.H.; Ye, Y.; Zhang, X.; Huang, Y.; Xing, L.; Zhou, Y.; Qin, H. An all-solid-state ammonium ion-selective electrode based on polyaniline as transducer and poly (o-phenylenediamine) as sensitive membrane. Int. J. Electrochem. Sci. 2016, 11, 9928–9940. [Google Scholar]
- Wu, R.; Salim, W.W.; Malhotra, S.; Brovont, A.; Park, J.; Pekarek, S.; Banks, M.K.; Porterfield, D.M. Self-powered mobile sensor for in-pipe potable water quality omnitoring. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Freiburg, Germany, 27–31 October 2013; pp. 14–16. [Google Scholar]
- Bakker, E.; Pretsch, E. Modern potentiometry. Angew. Chem. Int. Ed. 2007, 46, 5660–5668. [Google Scholar] [CrossRef] [PubMed]
- Benoudjit, A.M.; Shohibuddin, I.U.S.; Bader, M.M.; Salim, W.W.A.W. Components of All-Solid-State Ion-Selective Electrodes (AS-ISEs). In Composite Materials: Applications in Engineering, Biomedicine and Food Science; Springer: Cham, Switzerland, 2020; pp. 351–366. [Google Scholar] [CrossRef]
- Cuartero, M.; Colozza, N.; Fernández-Pérez, B.M.; Crespo, G.A. Why ammonium detection is particularly challenging but insightful with ionophore-based potentiometric sensors–an overview of the progress in the last 20 years. Analyst 2020, 145, 3188–3210. [Google Scholar] [CrossRef] [PubMed]
- Benoudjit, A.; Bader, M.M.; Salim, W.W.A.W. Study of electropolymerized PEDOT: PSS transducers for application as electrochemical sensors in aqueous media. Sens. Bio-Sens. Res. 2018, 17, 18–24. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors based on conducting polymers. Electroanal. Int.l J. Devot. Fundam. Pract. Aspects Electroanal. 2003, 15, 366–374. [Google Scholar]
- Bobacka, J. Conducting polymer-based solid-state ion-selective electrodes. Electroanal. Int. J. Devot. Fundam. Pract. Aspects Electroanal. 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Sayyah, S.M.; El-Deeb, M.M.; Kamal, S.M.; Azooz, R.E. Electropolymerization of o-phenylenediamine on Pt-electrode from aqueous acidic solution: Kinetic, mechanism, electrochemical studies and characterization of the polymer obtained. J. Appl. Polym. Sci. 2009, 112, 3695–3706. [Google Scholar] [CrossRef]
- Pine research; Screen-Printed Electrode Information-Carbon and Ceramic Electrode Information. Pine Res. Instrum. 2016, 10036, 1–10.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benoudjit, A.; Abd-Wahab, M.F.; Salim, W.W.A.W. Fabrication of an All-Solid-State Ammonium Ion–Selective Electrode by a Two-Step Process Using Cyclic Voltammetry. Proceedings 2020, 60, 12. https://doi.org/10.3390/IECB2020-07057
Benoudjit A, Abd-Wahab MF, Salim WWAW. Fabrication of an All-Solid-State Ammonium Ion–Selective Electrode by a Two-Step Process Using Cyclic Voltammetry. Proceedings. 2020; 60(1):12. https://doi.org/10.3390/IECB2020-07057
Chicago/Turabian StyleBenoudjit, Abdelmohsen, Mohd. Firdaus Abd-Wahab, and Wan Wardatul Amani Wan Salim. 2020. "Fabrication of an All-Solid-State Ammonium Ion–Selective Electrode by a Two-Step Process Using Cyclic Voltammetry" Proceedings 60, no. 1: 12. https://doi.org/10.3390/IECB2020-07057
APA StyleBenoudjit, A., Abd-Wahab, M. F., & Salim, W. W. A. W. (2020). Fabrication of an All-Solid-State Ammonium Ion–Selective Electrode by a Two-Step Process Using Cyclic Voltammetry. Proceedings, 60(1), 12. https://doi.org/10.3390/IECB2020-07057




