Closed Irreversible Cycles Analysis Based on Finite Physical Dimensions Thermodynamics †
Abstract
:1. Introduction
2. The Irreversible Energy Efficiency—The Number of Internal Irreversibility
- ✓
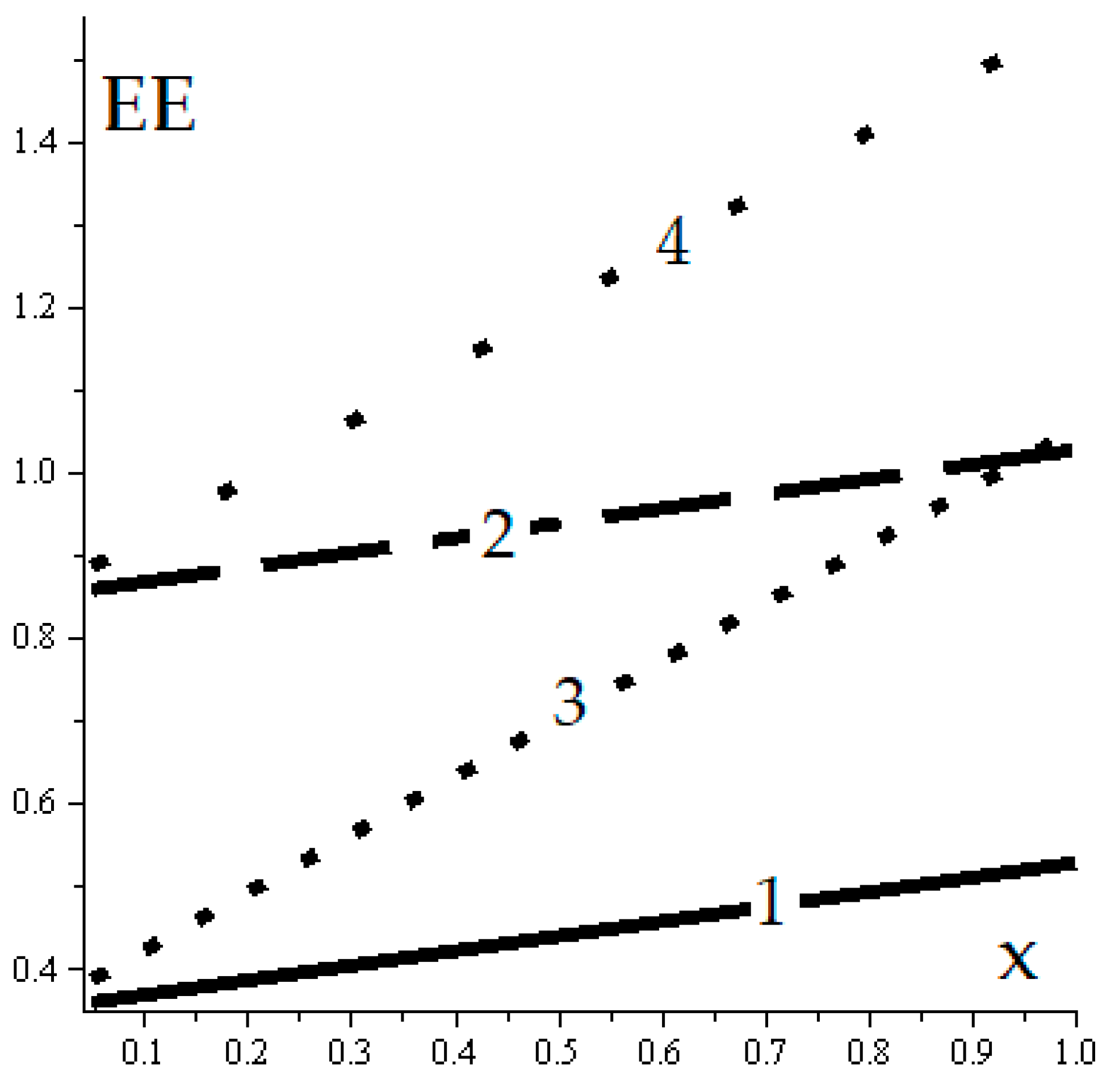
- Basic closed engine cycle, see Figure 1
- The reference specific entropy during the cyclic heating
- The specific heat inputwhere Tmq is the mean thermodynamic temperature of reversible heating process.
- The specific entropy variation during the cyclic cooling
- The specific heat outputwhere Tmq0 is the mean thermodynamic temperature of reversible cooling process
- The number of internal irreversibility (Nirr) and the irreversible energy efficiency (EEirr)where w is the specific useful work. When respectively it is re-obtained the energy efficiency of the endoreversible basic closed engine cycle. The number of internal irreversibility might ensure the needed link between the external heat reservoirs parameters (constant or variable) by the intermediary of the internal overall irreversibility.
- ✓
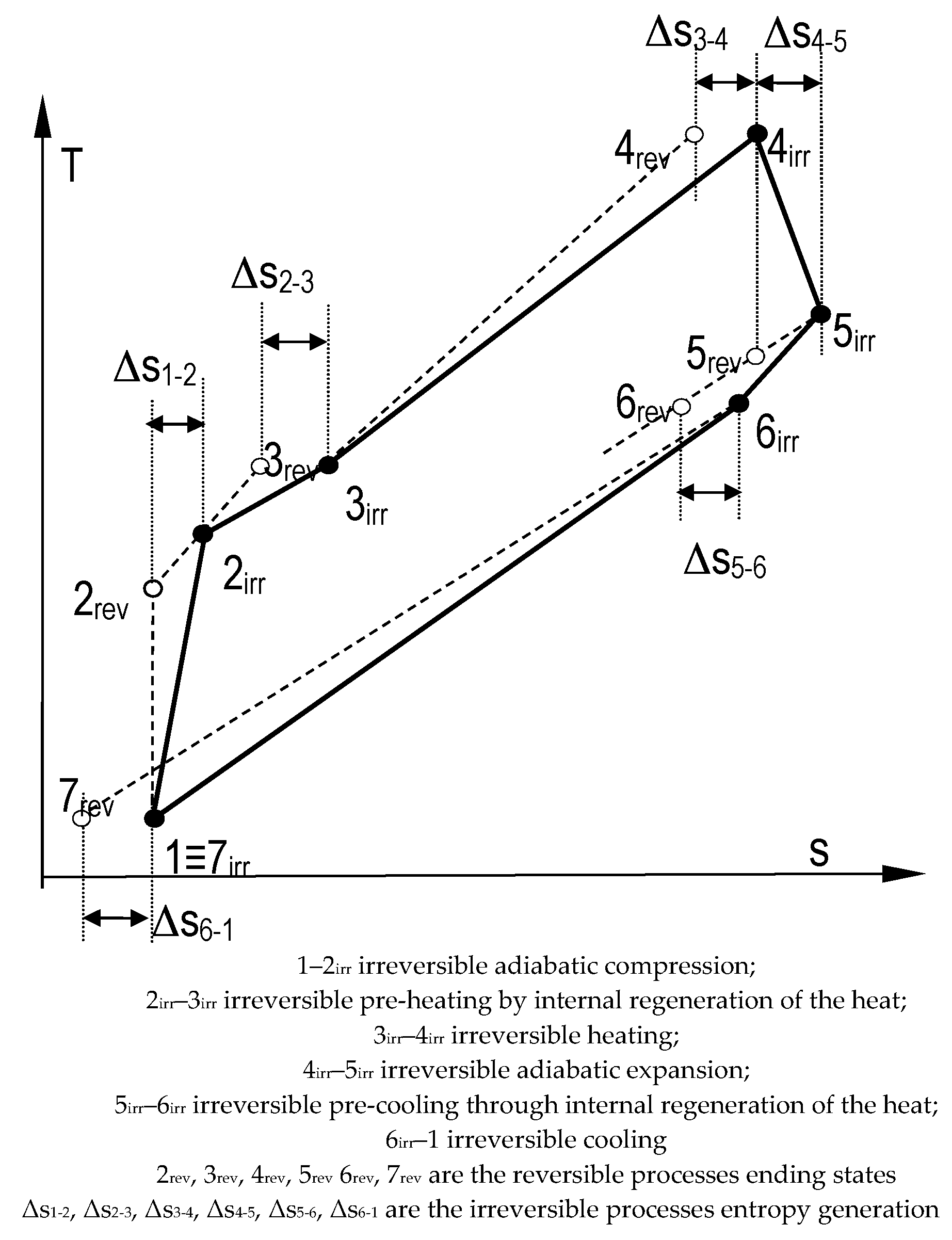
- Closed engine cycle with internal regeneration of the heat, see Figure 2
- The reference specific entropy during the cyclic heating
- The specific heat inputwhere Tmq is the mean thermodynamic temperature of reversible heating process.
- The specific entropy variation during the cyclic coolingwhere Δsreg is the entropy variation during the pre-heating process 2irr–3rev, Treg is the mean thermodynamic temperature of the pre-heating process 2irr–3rev, T*reg is the mean thermodynamic temperature of the pre-cooling process 5irr–6rev.
- The specific heat outputwhere Tmq0 is the mean thermodynamic temperature of reversible cooling process
- The number of internal irreversibility (Nirr) and the irreversible energy efficiency (EEirr)where w is the specific useful work. When . respectively, , , it is re-obtained the energy efficiency of the endoreversible closed engine cycle with internal regeneration of the heat. The number of internal irreversibility might ensure the needed link between the external heat reservoirs parameters (constant or variable) by the intermediary of the internal overall irreversibility.
- ✓
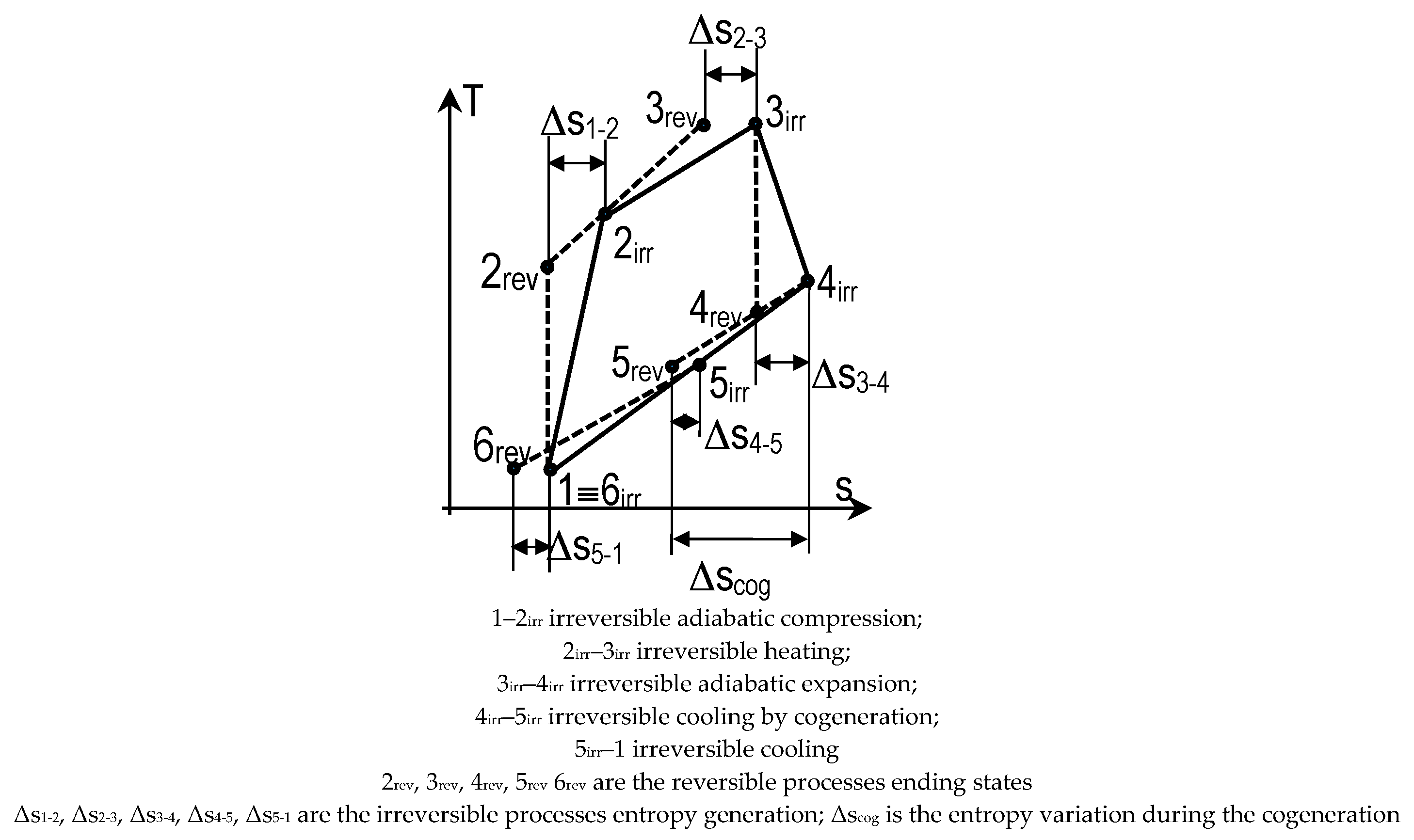
- Closed cogeneratione cycle, see Figure 3
- The reference specific entropy during the cyclic heating
- The specific heat inputwhere Tmq is the mean thermodynamic temperature of reversible heating process.
- The specific entropy variation during the cyclic coolingwhere Δscog = s5rev–s4irr < 0 is the entropy variation during the cogeneration (cooling process) 4irr–5rev.
- The number of internal irreversibility (Nirr,cog)
- The specific heat outputwhere Tmq0 is the mean thermodynamic temperature of reversible cooling process.
- The specific useful heat by cogeneration (qcog) and the irreversible useful power (w)
- The irreversible energy efficiency (EEirr)where . When respectively, , it is re-obtained the energy efficiency of the endoreversible closed cogeneration cycle correspondingly, it is equalizing the unity. The number of internal irreversibility might ensure the needed link between the external heat reservoirs parameters (constant or variable) by the intermediary of the internal overall irreversibility.
- ✓
- Basic closed refrigeration cycle, see Figure 4
- The reference specific entropy during the cyclic heating
- The specific heat inputwhere Tmq is the mean thermodynamic temperature of reversible heating process.
- The specific entropy variation during the cyclic cooling
- The specific heat outputwhere Tmq0 is the mean thermodynamic temperature of reversible cooling process.
- The number of internal irreversibility (Nirr) and the irreversible energy efficiency (EEirr)where COP is the coefficient of performance of refrigeration cycles and w is the specific consumed work. When respectively it is re-obtained the energy efficiency of the endoreversible basic refrigeration engine cycle. The number of internal irreversibility might ensure the needed link between the external heat reservoirs parameters (constant or variable) by the intermediary of the internal overall irreversibility.
3. The Design Imposed Operational Conditions
- constant reference entropy, almost a theoretical approach,
- constant specific power, when the client requires this,
- constant energy efficiency, when we follow the maximum possible energy efficiency, and
- constant heat input when we have limited heat resources.
- Constant specific reference entropy: kJ/kg.K
- Constant specific heat input: kJ/kg
- Constant specific power output: kJ/kg
- Constant energy efficiency:
4. The Irreversible Trigeneration Cycles Design Based on Finite Physical Dimensions Thermodynamics
- Supplying power and refrigeration rate, the summer season;
- Supplying power and heat rate by engine cycle, and refrigeration rate by reverse cycle, the winter season;
- Supplying power by engine cycle, and heat and refrigeration rates by reverse cycle, the winter season; and
- Supplying power and heat rate by engine cycle, and heat and refrigeration rates by reverse cycle, the winter season.
- two mean log temperature differences between the working fluids and external heat sources;
- two dimensionless thermal conductance inventories, as in [15];
- two extra restrictive parameters (adopted energy efficiency of engine and of refrigeration machine).
4.1. Basic Mathematical Model
4.1.1. Engine Irreversible Cycle
- The reference entropy rate for the irreversible engine cycle is, see Figure 1:
- The ratio of external heat reservoirs temperature , and
- The mean log temperature difference ΔTH [K], inside the heat exchanger allocated to the hot side of engine, having the thermal conductance .
- Thermal conductance inventory:where U [kW·m–2·K–1] is the overall heat transfer coefficient and A [m2] is the heat transfer area.
- Energy balance equations:where
- −
- [kg·s–1] is the working fluid mass flow rate through engine;
- −
- [kW] is the input heat rate;
- −
- THS [K] is the mean temperature of the heat source [15];
- −
- [K] is the cycle mean thermodynamic temperature at the hot side;
- −
- [kW] is the exhaust heat rate;
- −
- TC [K] is the cycle mean thermodynamic temperature at the cold side;
- −
- [K] is the mean temperature of the heat sink [15];
- −
- [kW] is the power and EEirr,E is the irreversible energy efficiency.
4.1.2. Refrigeration Irreversible Cycle
- The reference entropy rate of working fluid for the irreversible reverse cycle is, see Figure 4:The finite physical dimension control parameters are:
- External heat reservoirs temperatures ratio ;
- Variable mean log temperature difference ΔTC [K], inside the heat exchanger at the heat source having the thermal conductance .
- Thermal conductance inventory:where U [kW·m–2·K–1] is the overall heat transfer coefficient and A [m2] is the heat transfer area.
- Energy balance equations:where
- −
- [kg·s–1] is the mass flow rate of the working fluid through the refrigeration machine;
- −
- [kW] is the refrigeration heat rate;
- −
- [K] is the mean thermodynamic temperature at the cycle cold side;
- −
- [K] is the fitting mean temperature of the cold source;
- −
- [kW] is the absolute heat rate at the heat sink;
- −
- [K] is mean thermodynamic temperature at the cycle hot side;
- −
- T0S is the fitting mean temperature of the heat sink.
4.2. Irreversible Trigeneration System
- Case “a”—energy efficiency:
- Case “b”—energy efficiency:
- Case “c”—energy efficiency:
- Case “d”—energy efficiency:
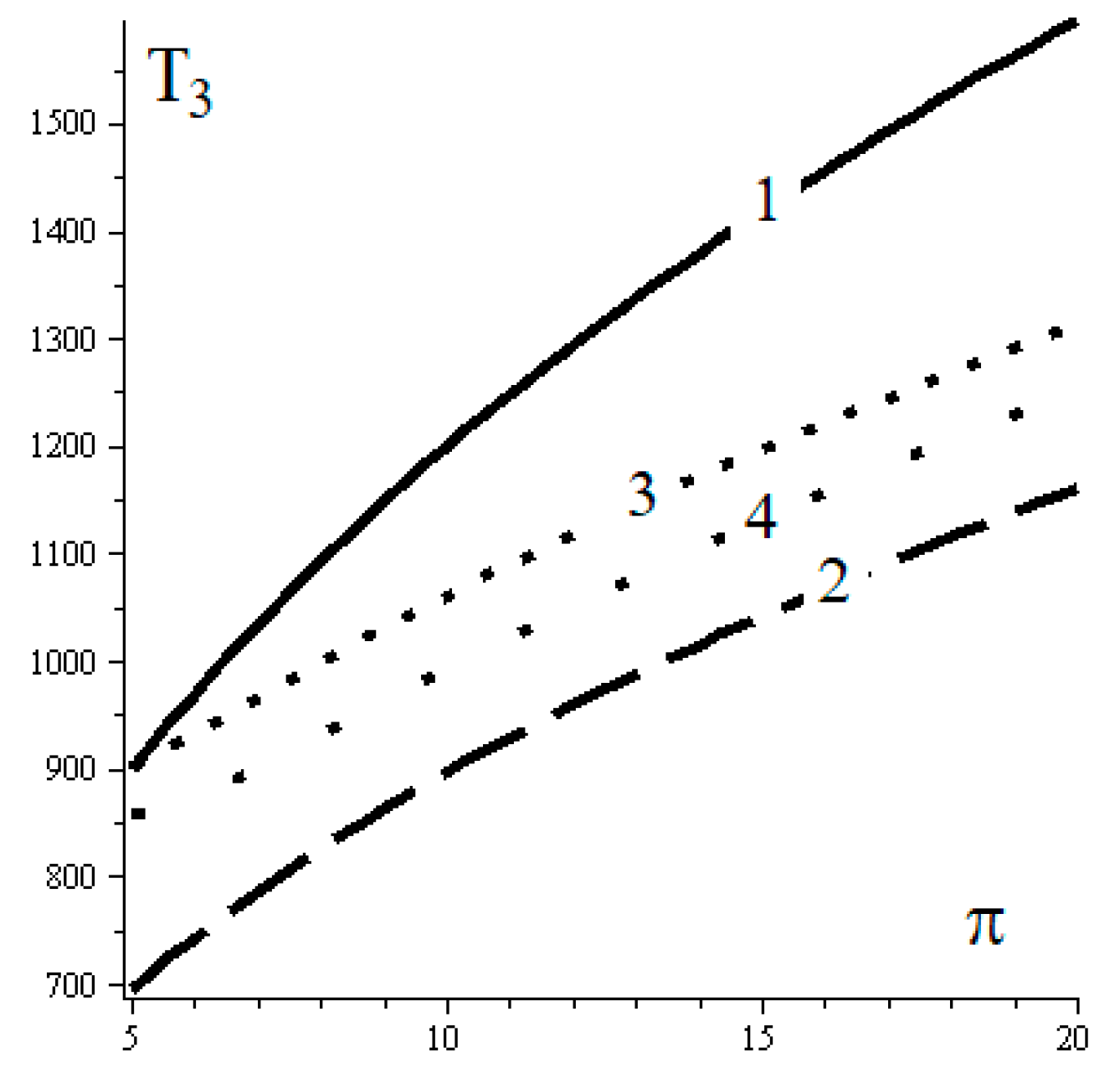
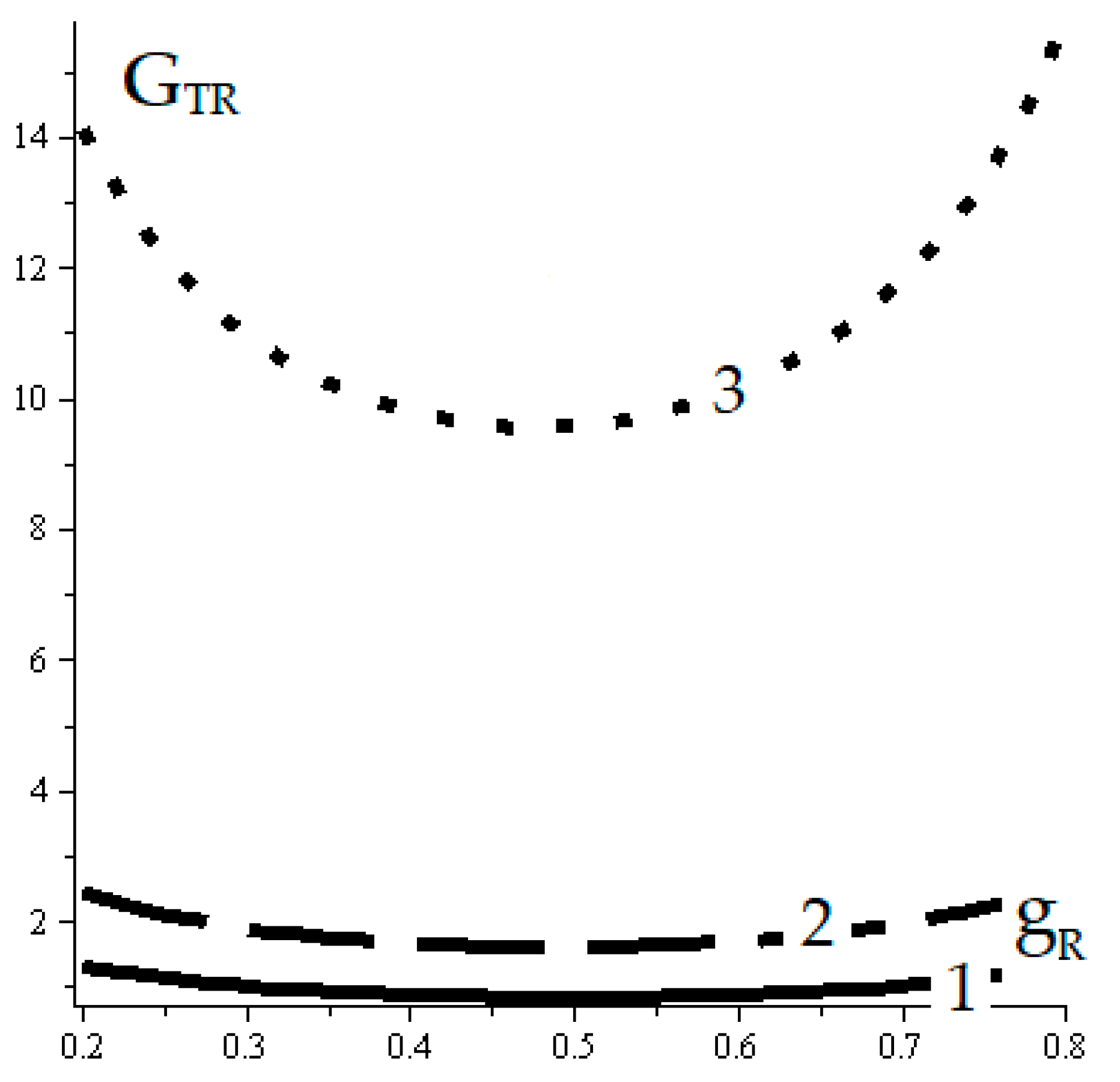
5. Discussion
- Defining the reference complete reversible trigeneration models by considering the Carnot cycle.
- Defining the reference endoreversible trigeneration models well completed through FPDT models: [15].
- Defining the corresponding reference irreversible generalizing models by FPDT assessments. This stage way is shortly described by this paper.
- Defining the optimization methods considering either thermodynamic, or CAPEX/CapEx – Capital Expenditure, or operational costs or mixed criteria.
- Defining the reference models for possible interconnected trigeneration grids and the evaluation and optimization methods.
6. Conclusions
Acknowledgments
References
- Ochoa, G.V.; Rojas, J.P.; Forero, J.D. Advance Exergo-Economic Analysis of a Waste Heat Recovery System Using ORC for a Bottoming Natural Gas Engine. Energies 2020, 13, 267. [Google Scholar] [CrossRef]
- Song, J.; Song, H.H. Analytical Approach to the Exergy Destruction and the Simple Expansion Work Potential in the Constant Internal Energy and Volume Combustion Process. Energies 2020, 13, 395. [Google Scholar] [CrossRef]
- Rangel-Hernandez, V.H.; Torres, C.; Zaleta-Aguilar, A.; Gomez-Martinez, M.A. The Exergy Costs of Electrical Power, Cooling, and Waste Heat from a Hybrid System Based on a Solid Oxide Fuel Cell and an Absorption Refrigeration System. Energies 2019, 12, 3476. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Wu, J.; Ma, C. Exergy and Exergoeconomic Analysis of a Combined Cooling, Heating, and Power System Based on Solar Thermal Biomass Gasification. Energies 2019, 12, 2418. [Google Scholar] [CrossRef]
- Guerra, J.P.; Cardoso, F.H.; Nogueira, A.; Kulay, L. Thermodynamic and Environmental Analysis of Scaling up Cogeneration Units Driven by Sugarcane Biomass to Enhance Power Exports. Energies 2018, 11, 73. [Google Scholar] [CrossRef]
- Almutairi, A.; Pilidis, P.; Al-Mutawa, N. Energetic and Exergetic Analysis of Combined Cycle Power Plant: Part-1 Operation and Performance. Energies 2015, 8, 14118–14135. [Google Scholar] [CrossRef]
- Nami, H.; Anvari-Moghaddam, A.; Arabkoohsar, A. Thermodynamic, Economic, and Environmental Analyses of a Waste-Fired Trigeneration Plant. Energies 2020, 13, 2476. [Google Scholar] [CrossRef]
- Tzivanidis, C.; Bellos, E. A Comparative Study of Solar-Driven Trigeneration Systems for the Building Sector. Energies 2020, 13, 2074. [Google Scholar] [CrossRef]
- Jamaluddin, K.; Alwi, S.R.W.; Hamzah, K.; Klemeš, J.J. A Numerical Pinch Analysis Methodology for Optimal Sizing of a Centralized Trigeneration System with Variable Energy Demands. Energies 2020, 13, 2038. [Google Scholar] [CrossRef]
- Bartnik, R.; Buryn, Z.; Hnydiuk-Stefan, A.; Skomudek, W.; Otawa, A. Thermodynamic and Economic Analysis of Trigeneration System Comprising a Hierarchical Gas-Gas Engine for Production of Electricity, Heat and Cold. Energies 2020, 13, 1006. [Google Scholar] [CrossRef]
- Sztekler, K.; Kalawa, W.; Mika, L.; Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Nowak, W.; Siwek, T.; Bieniek, A. Modeling of a Combined Cycle Gas Turbine Integrated with an Adsorption Chiller. Energies 2020, 13, 515. [Google Scholar] [CrossRef]
- Villarroel-Schneider, J.; Malmquist, A.; Araoz, J.A.; Martí-Herrero, J.; Martin, A. Performance Analysis of a Small-Scale Biogas-Based Trigeneration Plant: An Absorption Refrigeration System Integrated to an Externally Fired Microturbine. Energies 2019, 12, 3830. [Google Scholar] [CrossRef]
- Dumitrascu, G. The way to optimize the irreversible cycles*. Termotehnica 2008, 2, 18–21, selected paper from COFRET’08, Juin 11–13, 2008, Nantes–France. [Google Scholar]
- Dumitrașcu, G.; Feidt, M.; Namat, A.R.J.; Grigorean, Ş.; Horbaniuc, B. Thermodynamic analysis of irreversible closed Brayton engine cycles used in trigeneration systems, The XXIInd National Conference on Thermodynamics with International Participation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 595, 012022. [Google Scholar] [CrossRef]
- Gheorghe, D.; Michel, F.; Aristotel, P.; Stefan, G. Endoreversible Trigeneration Cycle Design Based on Finite Physical Dimensions Thermodynamics. Energies 2019, 12, 3165. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrascu, G.; Feidt, M.; Grigorean, S. Closed Irreversible Cycles Analysis Based on Finite Physical Dimensions Thermodynamics. Proceedings 2020, 58, 37. https://doi.org/10.3390/WEF-06905
Dumitrascu G, Feidt M, Grigorean S. Closed Irreversible Cycles Analysis Based on Finite Physical Dimensions Thermodynamics. Proceedings. 2020; 58(1):37. https://doi.org/10.3390/WEF-06905
Chicago/Turabian StyleDumitrascu, Gheorghe, Michel Feidt, and Stefan Grigorean. 2020. "Closed Irreversible Cycles Analysis Based on Finite Physical Dimensions Thermodynamics" Proceedings 58, no. 1: 37. https://doi.org/10.3390/WEF-06905
APA StyleDumitrascu, G., Feidt, M., & Grigorean, S. (2020). Closed Irreversible Cycles Analysis Based on Finite Physical Dimensions Thermodynamics. Proceedings, 58(1), 37. https://doi.org/10.3390/WEF-06905




