Influence of Sex and Diet on the Gastrointestinal Tract in a Mice Model with Partial Deficiency for TGF-β3 †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Experimental Animals and Groups
2.3. Functional Study of the Gastrointestinal Transit
2.4. Structural Study: Macroscopic Study of the Gastrointestinal Organs
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. Functional Study of Gastrointestinal Motor Function—Phase 1: Characteristics of Excreted Feces
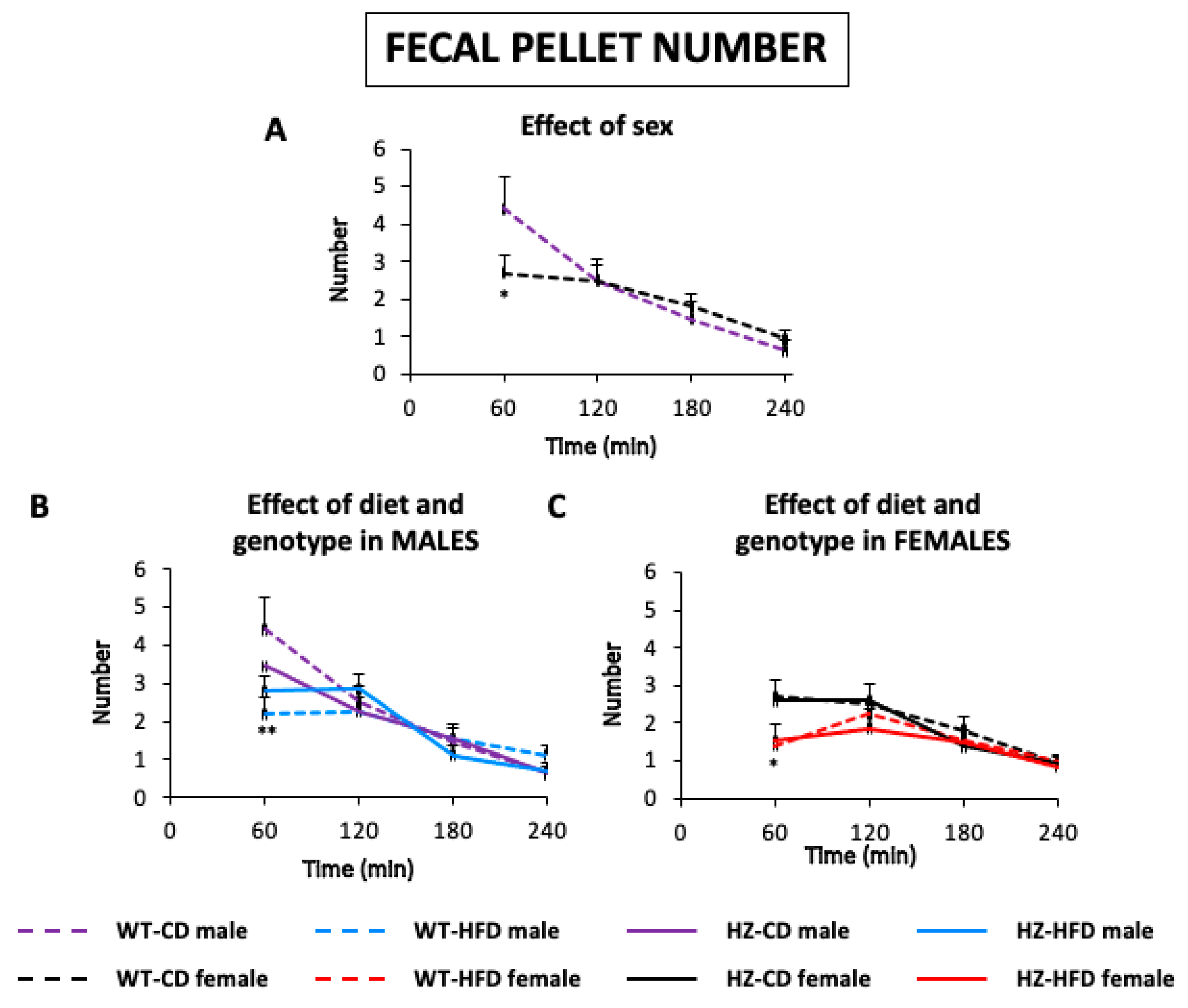
3.1.1. Number of Feces
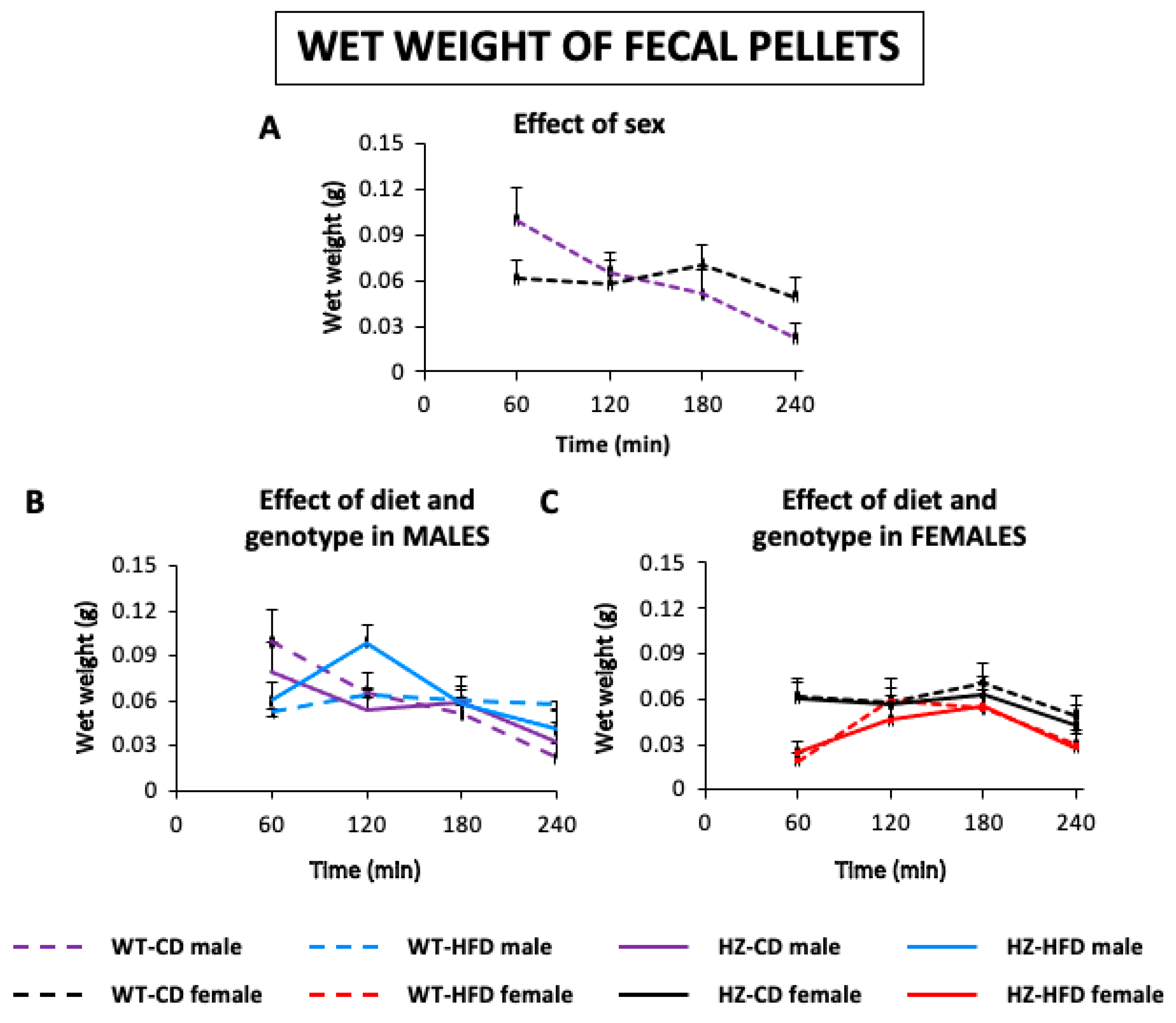
3.1.2. Wet Weight of Feces
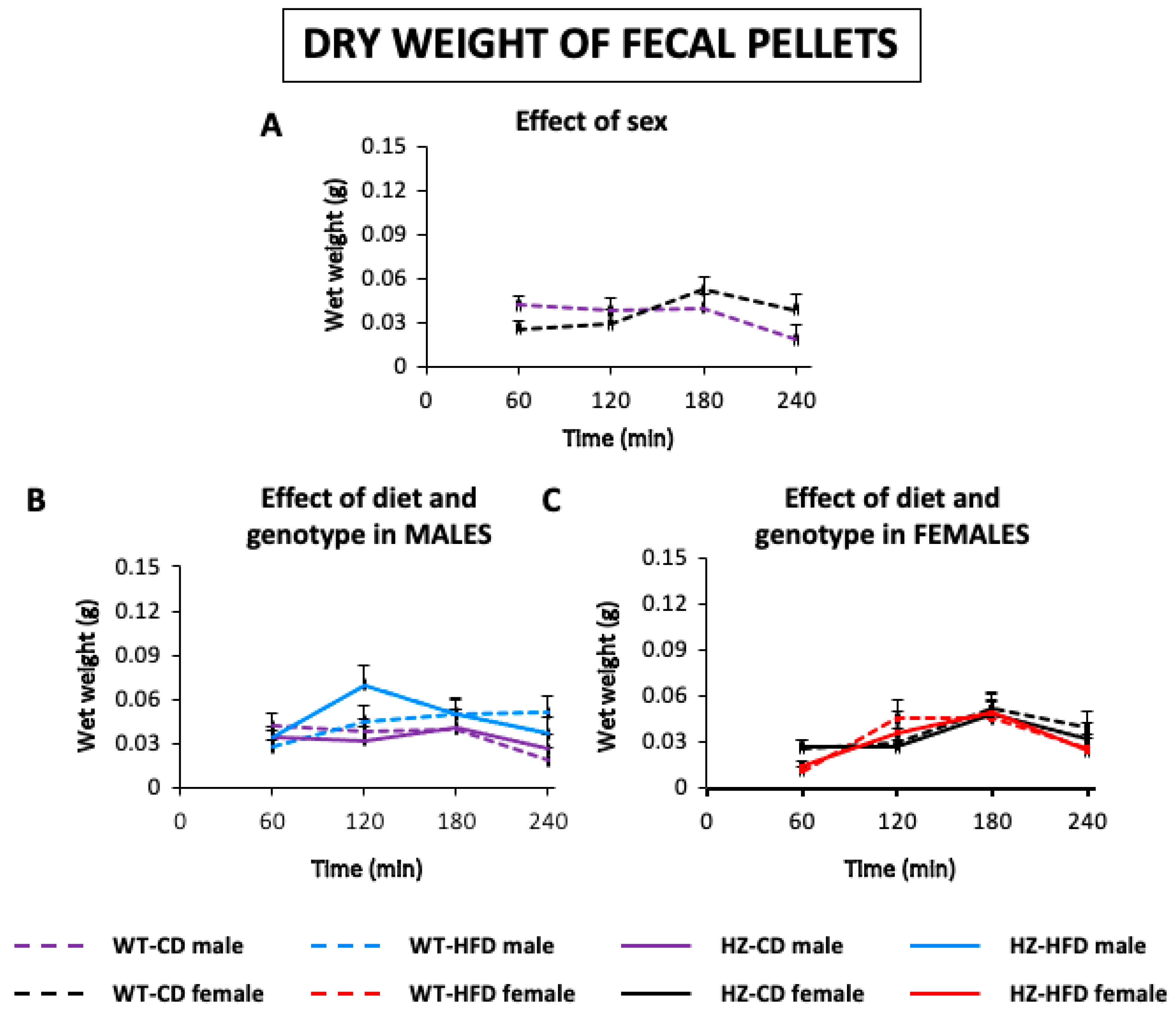
3.1.3. Dry Weight of Feces
3.1.4. Fecal Moisture
3.2. Functional Study of Gastrointestinal Motor Function—Phase 2: Gastrointestinal Transit
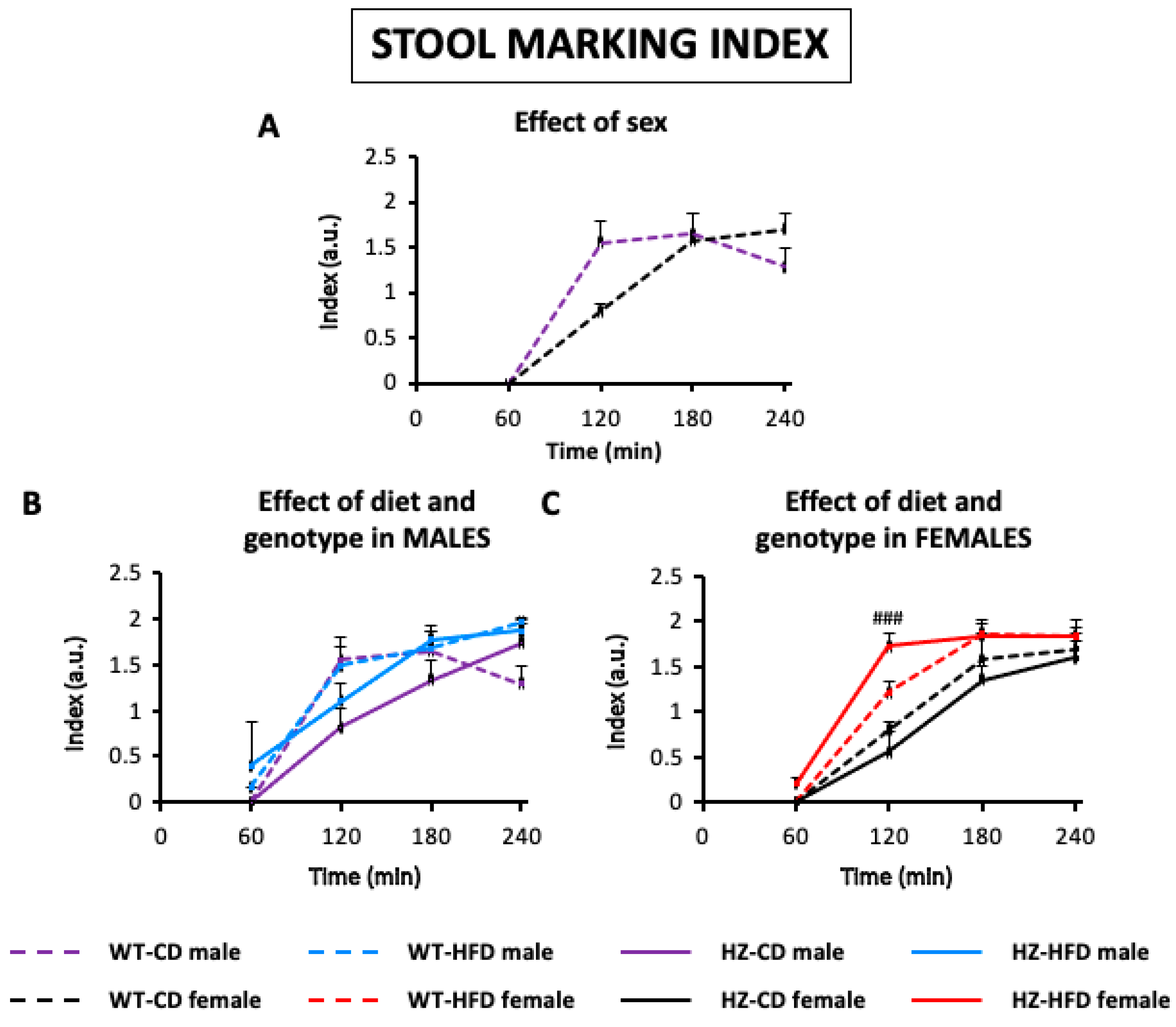
3.2.1. Stool Marking Index
3.2.2. Latency of Expulsion of the First Marked Fecal Pellet
3.3. Structural Study: Macroscopic Study of the Gastrointestinal Organs
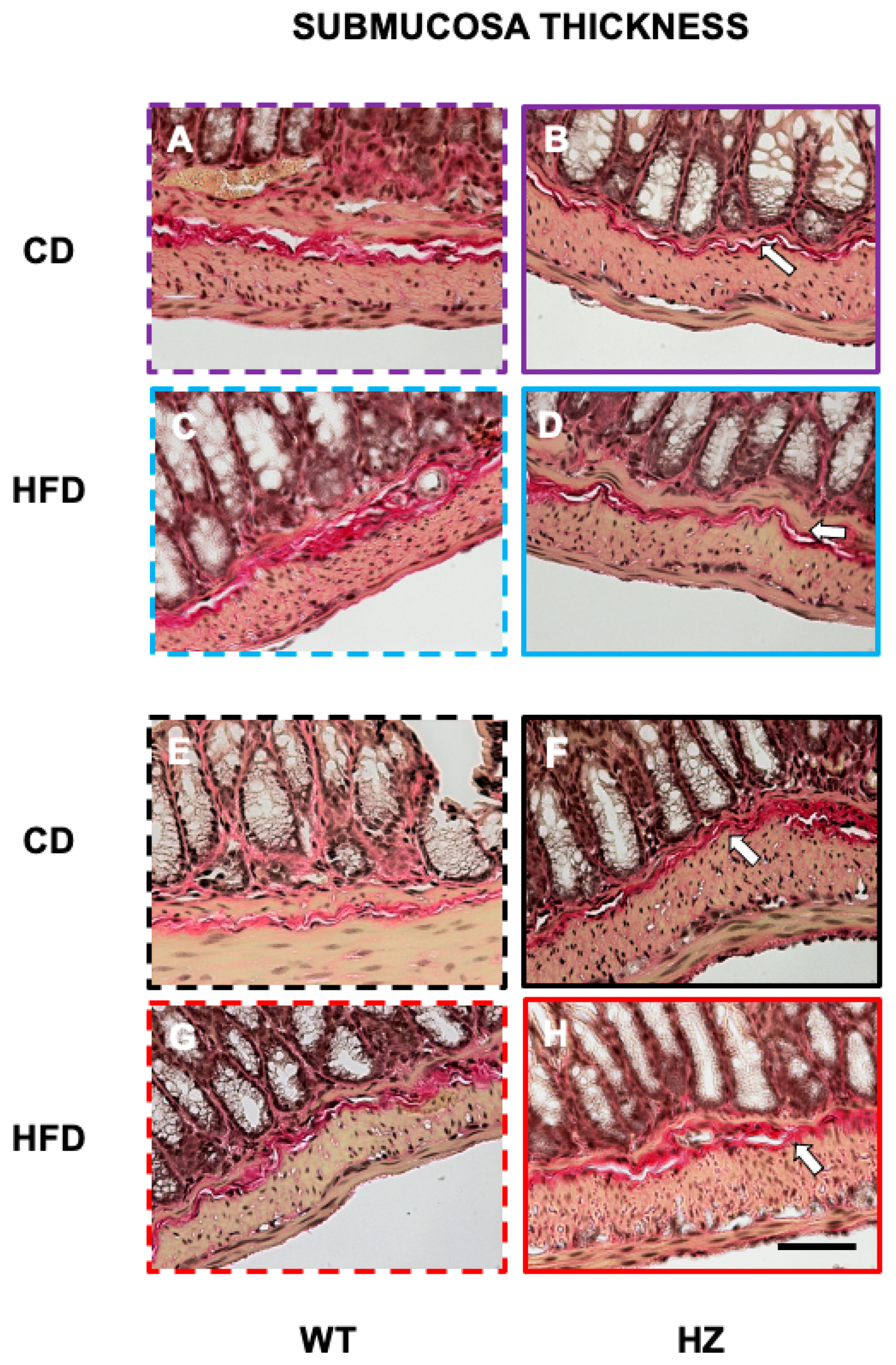
3.4. Structural Study: Microscopic Study of Distal Colon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, J.S.; Liu, C.; Derynck, R. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 2009, 19, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, H.J.; Kim, S.J.; Hahm, K.B. Connection between inflammation and carcinogenesis in gastrointestinal tract: Focus on TGF-β signaling. World J. Gastroenterol. 2010, 16, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Laverty, H.G.; Wakefield, L.M.; Occleston, N.L.; O’Kane, S.; Ferguson, M.W.J. TGF-β3 and cancer: A review. Cytokine Growth Factor Rev. 2009, 20, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Al-Sammarraie, N.; Gebere, M.G.; Bhattacharya, A.; Chopra, S.; Johnson, J.; Peña, E.A.; Eberth, J.F.; Poelmann, R.E.; de Gittenberger-Groot, A.C.; et al. Transforming Growth Factor Beta3 is Required for Cardiovascular Development. J. Cardiovasc. Dev. Dis. 2020, 7, 19. [Google Scholar] [CrossRef]
- Kaartinen, V.; Voncken, J.W.; Shuler, C.; Warburton, D.; Bu, D.; Heisterkamp, N.; Groffen, J. Abnormal lung development and cleft palate in mice lacking TGF–β3 indicates defects of epithelial–mesenchymal interaction. Nat. Genet. 1995, 11, 415–421. [Google Scholar] [CrossRef]
- Proetzel, G.; Pawlowski, S.A.; Wiles, M.V.; Yin, M.; Boivin, G.P.; Howles, P.N.; Ding, J.; Ferguson, M.W.J.; Doetschman, T. Transforming growth factor–β3 is required for secondary palate fusion. Nat. Genet. 1995, 11, 409–414. [Google Scholar] [CrossRef]
- Petrus, P.; Mejhert, N.; Corrales, P.; Lecoutre, S.; Li, Q.; Maldonado, E.; Kulyté, A.; Lopez, Y.; Campbell, M.; Acosta, J.R.; et al. Transforming Growth Factor-β3 Regulates Adipocyte Number in Subcutaneous White Adipose Tissue. Cell Rep. 2018, 25, 551–560.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Fragoulis, G.E. Inflammation, obesity and rheumatic disease: Common mechanistic links. A narrative review. Ther. Adv. Musculoskelet. Dis. 2018, 10, 157–167. [Google Scholar] [CrossRef]
- Ihara, S.; Hirata, Y.; Koike, K. TGF-β in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. J. Gastroenterol. 2017, 52, 777–787. [Google Scholar] [CrossRef]
- Vallance, B.A.; Gunawan, M.I.; Hewlett, B.; Bercik, P.; Van Kampen, C.; Galeazzi, F.; Sime, P.J.; Gauldie, J.; Collins, S.M. TGF-β1 gene transfer to the mouse colon leads to intestinal fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289. [Google Scholar] [CrossRef] [PubMed]
- Sans, M.; Masamunt, M.C. Fibrogenesis and inflammatory bowel disease. Gastroenterol. Hepatol. 2007, 30, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, I.C.; Maxwell, L.; Doe, W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm. Bowel Dis. 2001, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. (Aust.) 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Uranga, J.A.; López-Miranda, V.; Lombó, F.; Abalo, R. Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. Pharmacol. Rep. 2016, 68, 816–826. [Google Scholar] [CrossRef]
- Gruber, L.; Kisling, S.; Lichti, P.; Martin, F.P.; May, S.; Klingenspor, M.; Lichtenegger, M.; Rychlik, M.; Haller, D. High Fat Diet Accelerates Pathogenesis of Murine Crohn’s Disease-Like Ileitis Independently of Obesity. PLoS ONE 2013, 8, e71661. [Google Scholar] [CrossRef]
- Emerenziani, S.; Guarino, M.P.L.; Asensio, L.M.T.; Altomare, A.; Ribolsi, M.; Balestrieri, P.; Cicala, M. Role of overweight and obesity in gastrointestinal disease. Nutrients 2020, 12, 111. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Squecco, R.; Baccari, M.C. Adipocytes-released Peptides Involved in the Control of Gastrointestinal Motility. Curr. Protein Pept. Sci. 2019, 20, 614–629. [Google Scholar] [CrossRef]
- Stavely, R.; Abalo, R.; Nurgali, K. Targeting Enteric Neurons and Plexitis for the Management of Inflammatory Bowel Disease. Curr. Drug Targets 2020, 21. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex differences in gut microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Houdeau, E. Sex Differences in Gastrointestinal Physiology and Diseases: From Endogenous Sex Hormones to Environmental Endocrine Disruptor Agents. Sex Differ. Physiol. 2016, 125–143. [Google Scholar] [CrossRef]
- Martínez-Álvarez, C.; Tudela, C.; Pérez-Miguelsanz, J.; O’Kane, S.; Puerta, J.; Ferguson, M.W.J. Medial edge epithelial cell fate during palatal fusion. Dev. Biol. 2000, 220, 343–357. [Google Scholar] [CrossRef]
- Babygirija, R.; Bülbül, M.; Cerjak, D.; Ludwig, K.; Takahashi, T. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci. Lett. 2011, 495, 77–81. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Donald, E.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Gastrointestinal dysfunction and enteric neurotoxicity following treatment with anticancer chemotherapeutic agent 5-fluorouracil. Neurogastroenterol. Motil. 2016, 28, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Girón, R.; Pérez-García, I.; Abalo, R. X-ray analysis of gastrointestinal motility in conscious mice. Effects of morphine and comparison with rats. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2016, 28, 74–84. [Google Scholar] [CrossRef]
- Barone, F.C.; Barton, M.E.; White, R.F.; Legos, J.J.; Kikkawa, H.; Shimamura, M.; Kuratani, K.; Kinoshita, M. Inhibition of phosphodiesterase type 4 decreases stress-induced defecation in rats and mice. Pharmacology 2007, 81, 11–17. [Google Scholar] [CrossRef]
- Liang, C.; Wang, K.Y.; Yu, Z.; Xu, B. Development of a novel mouse constipation model. World J. Gastroenterol. 2016, 22, 2799–2810. [Google Scholar] [CrossRef]
- Andrews, P.L.R.; Horn, C.C. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton. Neurosci. Basic Clin. 2006, 125, 100–115. [Google Scholar] [CrossRef]
- Horn, C.C.; Kimball, B.A.; Wang, H.; Kaus, J.; Dienel, S.; Nagy, A.; Gathright, G.R.; Yates, B.J.; Andrews, P.L.R. Why Can’t Rodents Vomit? A Comparative Behavioral, Anatomical, and Physiological Study. PLoS ONE 2013, 8, e60537. [Google Scholar] [CrossRef]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation. Medicine (U.S.) 2018, 97, e10631. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Kim, Y.W.; Park, S.Y.; Kim, J.Y. Estrogen rather than progesterone cause constipation in both female and male mice. Korean J. Physiol. Pharmacol. 2013, 17, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Reichardt, F.; Tabatabavakili, S.; Nezami, B.G.; Chassaing, B.; Mwangi, S.; Vijay-Kumar, M.; Gewirtz, A.; Srinivasan, S. Intestinal Dysbiosis Contributes to the Delayed Gastrointestinal Transit in High-Fat Diet Fed Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 328–339. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Fried, D.; Gulbransen, B.; Kadrofske, M.; Fernandes, R.; Xu, H.; Galligan, J. High-fat diet-induced obesity alters nitric oxide-mediated neuromuscular transmission and smooth muscle excitability in the mouse distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G210–G220. [Google Scholar] [CrossRef] [PubMed]
- Miron, I.; Dumitrascu, D.L. Gastrointestinal motility disorders in obesity. Acta Endocrinol. 2019, 15, 497–504. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Soares, A.; Beraldi, E.J.; Ferreira, P.E.B.; Bazotte, R.B.; Buttow, N.C. Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice. BMC Gastroenterol. 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, F.; Di, W.; Lv, Y.; Xia, F.; Sheng, Y.; Yu, J.; Ding, G. Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice. Mol. Med. Rep. 2020, 21, 1133–1144. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ren, J.; Hu, Q.; Deng, Y.; Chen, G.; Guo, K.; Li, R.; Li, Y.; Wu, L.; Wang, G.; et al. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-β signaling in a murine colitis model. Biochem. Pharmacol. 2016, 117, 57–67. [Google Scholar] [CrossRef] [PubMed]

| WT-CD | WT-HFD | HZ-CD | HZ-HFD | |
|---|---|---|---|---|
| Males | 107.5 ± 9.4 | 122.3 ± 9.9 | 147.3 ± 10.3 * | 107.1 ± 8.1 |
| Females | 138.5 ± 5.4 ^ | 120 ± 14 | 132 ± 7.6 | 135 ± 14.5 |
| WT-CD | WT-HFD | HZ-CD | HZ-HFD | |||
|---|---|---|---|---|---|---|
| Weight of organs at sacrifice | Stomach | Males | 0.35 ± 0.02 | 0.29 ± 0.02 | 0.38 ± 0.03 | 0.31 ±0.02 |
| Females | 0.36 ± 0.03 | 0.25 ± 0.02 ** | 0.4 ± 0.01 | 0.24 ±0.02 ### | ||
| Full small intestine | Males | 1.17 ± 0.03 | 1.14 ± 0.03 | 1.28 ± 0.06 | 1.24 ± 0.05 | |
| Females | 1.16 ± 0.02 | 1.03 ± 0.03 * | 1.26 ± 0.04 | 1.04 ± 0.02 ### | ||
| Cecum | Males | 0.43 ± 0.03 | 0.24 ± 0.01 *** | 0.48 ± 0.04 | 0.26 ± 0.01 ### | |
| Females | 0.53± 0.03 ^^ | 0.2 ± 0.01 *** | 0.56 ± 0.02 | 0.2 ± 0.01 ### | ||
| Full colorectum | Males | 0.35 ± 0.02 | 0.27 ± 0.02 * | 0.37 ± 0.02 | 0.31 ± 0.02 | |
| Females | 0.34 ± 0.02 | 0.24 ± 0.01 ** | 0.37 ± 0.03 | 0.24 ± 0.01 ### | ||
| Area or length of organs at sacrifice | Stomach | Males | 0.89 ± 0.05 | 0.81 ± 0.03 | 1 ± 0.05 | 0.85 ± 0.03 |
| Females | 0.91 ±0.05 | 0.81 ± 0.03 | 1.02 ± 0.04 | 0.8 ± 0.04 ## | ||
| Full small intestine | Males | 27.89 ± 0.68 | 25.83 ± 1.16 | 27.16 ± 0.77 | 24.7 ± 0.88 | |
| Females | 25.13 ± 0.45 ^^ | 24.72 ± 0.61 | 25.54 ± 0.4 | 24.59 ± 0.78 | ||
| Cecum | Males | 1.24 ± 0.07 | 0.68 ± 0.05 *** | 1.22 ± 0.09 | 0.82 ± 0.05 ### | |
| Females | 1.38 ± 0.04 | 0.63 ± 0.02 *** | 1.39 ± 0.05 | 0.62 ± 0.03 ### | ||
| Full colorectum | Males | 4.61 ± 0.15 | 3.95 ± 0.16 ** | 4.73 ± 0.12 | 4.01 ± 0.11 ## | |
| Females | 4.24 ± 0.08 | 3.93 ± 0.06 | 4.73 ± 0.15 * | 3.99 ± 0.13 ### | ||
| WT-CD | WT-HFD | HZ-CD | HZ-HFD | |
|---|---|---|---|---|
| Males | 7.32 ± 0.16 | 8.07 ± 0.28 | 5.92 ± 0.42 * | 6.74 ± 0.37 |
| Females | 6.79 ± 0.57 | 8.01 ± 0.73 | 8.94 ± 0.44 * | 9.60 ± 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, P.; Bagüés, A.; Escasany, E.; López-Tofiño, Y.; Márquez, A.; Medina-Gomez, G.; Uranga, J.A.; Abalo, R. Influence of Sex and Diet on the Gastrointestinal Tract in a Mice Model with Partial Deficiency for TGF-β3. Proceedings 2020, 61, 18. https://doi.org/10.3390/IECN2020-06983
Gallego P, Bagüés A, Escasany E, López-Tofiño Y, Márquez A, Medina-Gomez G, Uranga JA, Abalo R. Influence of Sex and Diet on the Gastrointestinal Tract in a Mice Model with Partial Deficiency for TGF-β3. Proceedings. 2020; 61(1):18. https://doi.org/10.3390/IECN2020-06983
Chicago/Turabian StyleGallego, Paula, Ana Bagüés, Elia Escasany, Yolanda López-Tofiño, Antonio Márquez, Gema Medina-Gomez, José Antonio Uranga, and Raquel Abalo. 2020. "Influence of Sex and Diet on the Gastrointestinal Tract in a Mice Model with Partial Deficiency for TGF-β3" Proceedings 61, no. 1: 18. https://doi.org/10.3390/IECN2020-06983
APA StyleGallego, P., Bagüés, A., Escasany, E., López-Tofiño, Y., Márquez, A., Medina-Gomez, G., Uranga, J. A., & Abalo, R. (2020). Influence of Sex and Diet on the Gastrointestinal Tract in a Mice Model with Partial Deficiency for TGF-β3. Proceedings, 61(1), 18. https://doi.org/10.3390/IECN2020-06983






