Optimization of Hetrotrophic Denitrification Using Glycerol as a Sustainable External Carbon Substrate †
Abstract
:1. Introduction
2. Results
2.1. Prefermenters (PF)
2.2. Denitrification
2.3. EBPR
2.4. Other Parameters
3. Discussion
4. Materials and Methods
4.1. Source of Materials
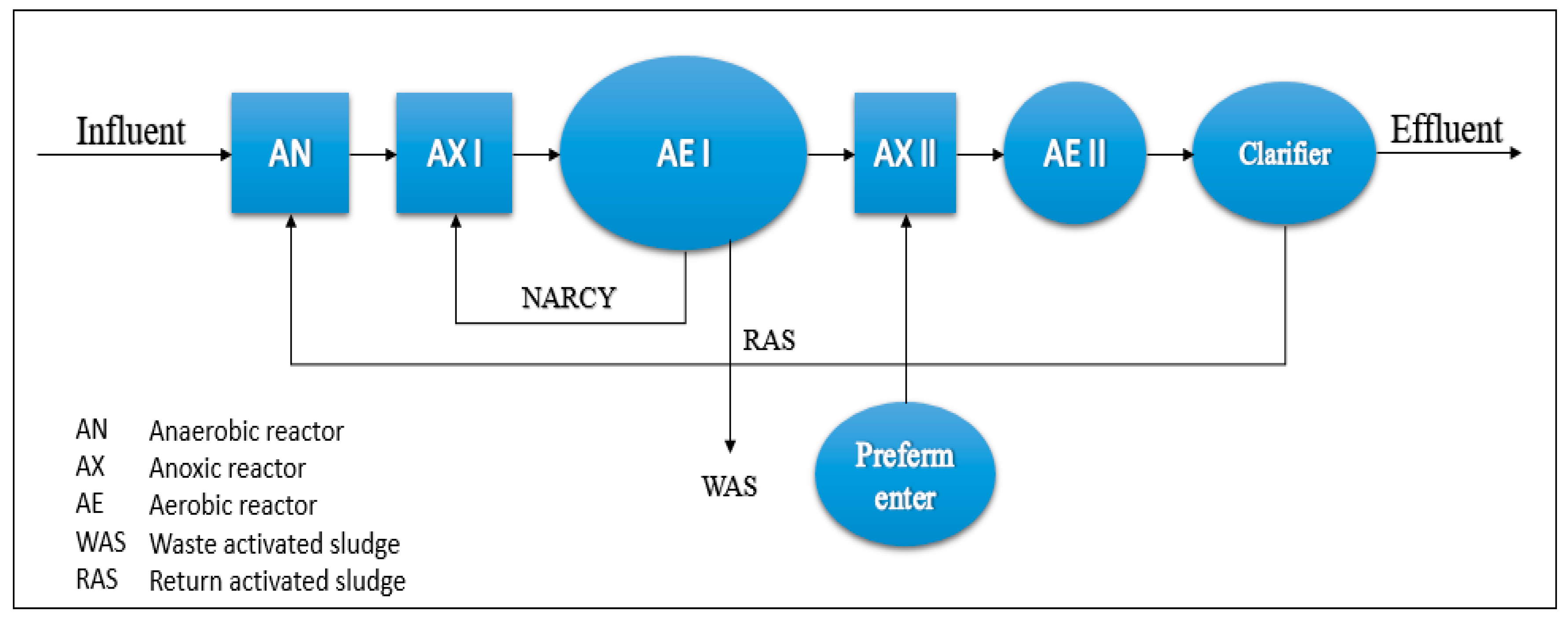
4.2. The Pilot Plant
4.3. Analytical Techniques
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BNR | Biological nutrient removal |
| EBPR | Enhanced biological phosphorus removal |
| HRT | Hydraulic retention time |
| MLSS | Mixed liquor suspended solids |
| MLVSS | Mixed liquor volatile suspended solids |
| NARCY | Nitrate recycle |
| NH3 | Ammonia |
| NOx | Nitrate + nitrite |
| PAOs | Polyphosphate accumulating organisms |
| RAS | Return activated sludge |
| SDR | Specific denitrification rate |
| SOP | Ortho-Phosphorus |
| SRT | Solid retention time |
| VFA | Volatile fatty acid |
References
- Metcalf&Eddy. Wastewater Engineering: Treatment and Resource Recovery; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Wu, C.-Y.; Peng, Y.Z.; Li, X.L.; Wang, S.Y. Effect of carbon source on biological nitrogen and phosphorus removal in an anaerobic-anoxic-oxic (A2O) process. J. Environ. Eng. 2010, 136, 1248–1254. [Google Scholar] [CrossRef]
- Bernat, K.; Kulikowska, D.; Godlewski, M. Crude glycerol as a carbon source at a low COD/N ratio provides efficient and stable denitritation. Desalin. Water Treat. 2016, 57, 19632–19641. [Google Scholar] [CrossRef]
- Shen, N.; Zhou, Y. Enhanced biological phosphorus removal with different carbon sources. Appl. Microbiol. Biotechnol. 2016, 100, 4735–4745. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Lim, B.R.; Cho, J.; Song, K.G.; Kim, K.P.; Ahn, K.H. Biological nitrogen and phosphorus removal and changes in microbial community structure in a membrane bioreactor: Effect of different carbon sources. Water Res. 2008, 42, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vazquez, C.M.; Oehmen, A.; Hooijmans, C.M.; Brdjanovic, D.; Gijzen, H.J.; Yuan, Z.; van Loosdrecht, M.C. Modeling the PAO–GAO competition: Effects of carbon source, pH and temperature. Water Res. 2009, 43, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Zeng, R.J.; Yuan, Z.; Keller, J. Anaerobic metabolism of propionate by polyphosphate-accumulating organisms in enhanced biological phosphorus removal systems. Biotechnol. Bioeng. 2005, 91, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Randall, A.A.; McCue, T. The efficiency of enhanced biological phosphorus removal from real wastewater affected by different ratios of acetic to propionic acid. Water Res. 2004, 38, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Merzouki, M.; Bernet, N.; Delgenes, J.P.; Benlemlih, M. Effect of prefermentation on denitrifying phosphorus removal in slaughterhouse wastewater. Bioresour. Technol. 2005, 96, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Kagawa, S.; Okamoto, S. Environmental and economic performance of a biodiesel plant using waste cooking oil. J. Clean. Prod. 2015, 101, 245–250. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel; Springer: London, UK, 2008. [Google Scholar]
- Grabińska-ńoniewska, A.; Słomczyński, T.; Kańska, Z. Denitrification studies with glycerol as a carbon source. Water Res. 1985, 19, 1471–1477. [Google Scholar] [CrossRef]
- Torà, J.A.; Baeza, J.A.; Carrera, J.; Oleszkiewicz, J.A. Denitritation of a high-strength nitrite wastewater in a sequencing batch reactor using different organic carbon sources. Chem. Eng. J. 2011, 172, 994–998. [Google Scholar] [CrossRef]
- Bodík, I.; BlŠťáková, A.; Sedláček, S.; Hutňan, M. Biodiesel waste as source of organic carbon for municipal WWTP denitrification. Bioresour. Technol. 2009, 100, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Sparling, R.; Lagasse, P.; Lee, Y.M.; Taniguchi, D.; Oleszkiewicz, J.A. Enhancing biological phosphorus removal with glycerol. Water Sci. Technol. 2010, 61, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Tayà, C.; Guisasola, A.; Baeza, J.A. Glycerol as a sole carbon source for enhanced biological phosphorus removal. Water Res. 2012, 46, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- McCue, T.; Naik, R.; Zepeda, M.; Liu, Y.H.; Vassiliev, I.; Randall, A.A. Changes in anoxic denitrification rate resulting from prefermentation of a septic, phosphorus-limited wastewater. Water Environ. Res. 2004, 76, 23–28. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995; p. 456. [Google Scholar]
| Reactor | Volume (V) | HRT (h) |
|---|---|---|
| AN | 3.6 | 1.6 |
| AX I | 5.9 | 2.6 |
| AE I | 18 | 7.8 |
| AX II | 3.3 | 1.4 |
| AE II | 0.8 | 0.3 |
| Total | 31.6 | 13.7 |
| Clarifier | 3.1 | 1.4 |
| PF | 10 | 5 |
| Combined Influent ** | |||
|---|---|---|---|
| Pilot A | Pilot B | ||
| TN | mg-N/L | 53.5 | 53.3 |
| NOx | * 0 | * 0 | |
| NH3 | 39.0 | 36.6 | |
| TP | mg-P/L | 6.0 | 5.8 |
| SOP | 4.7 | 4.3 | |
| TSS | mg/L | 287 | 270 |
| s-COD | 265 | 226 | |
| TCOD | 393 | 400 | |
| VFA | mg-COD/L | 44.0 | 88.3 |
| DO | mg/L | 0.08 | |
| PH | 7.5 ± 0.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamah, S.; Randall, A. Optimization of Hetrotrophic Denitrification Using Glycerol as a Sustainable External Carbon Substrate. Proceedings 2020, 48, 23. https://doi.org/10.3390/ECWS-4-06435
Salamah S, Randall A. Optimization of Hetrotrophic Denitrification Using Glycerol as a Sustainable External Carbon Substrate. Proceedings. 2020; 48(1):23. https://doi.org/10.3390/ECWS-4-06435
Chicago/Turabian StyleSalamah, Sultan, and Andrew Randall. 2020. "Optimization of Hetrotrophic Denitrification Using Glycerol as a Sustainable External Carbon Substrate" Proceedings 48, no. 1: 23. https://doi.org/10.3390/ECWS-4-06435
APA StyleSalamah, S., & Randall, A. (2020). Optimization of Hetrotrophic Denitrification Using Glycerol as a Sustainable External Carbon Substrate. Proceedings, 48(1), 23. https://doi.org/10.3390/ECWS-4-06435




