CuFe2O4@CuO: A Magnetic Composite Synthesized by Ultrasound Irradiation and Degradation of Methylene Blue on Its Surface in the Presence of Sunlight †
Abstract
1. Introduction
2. Results and Discussion
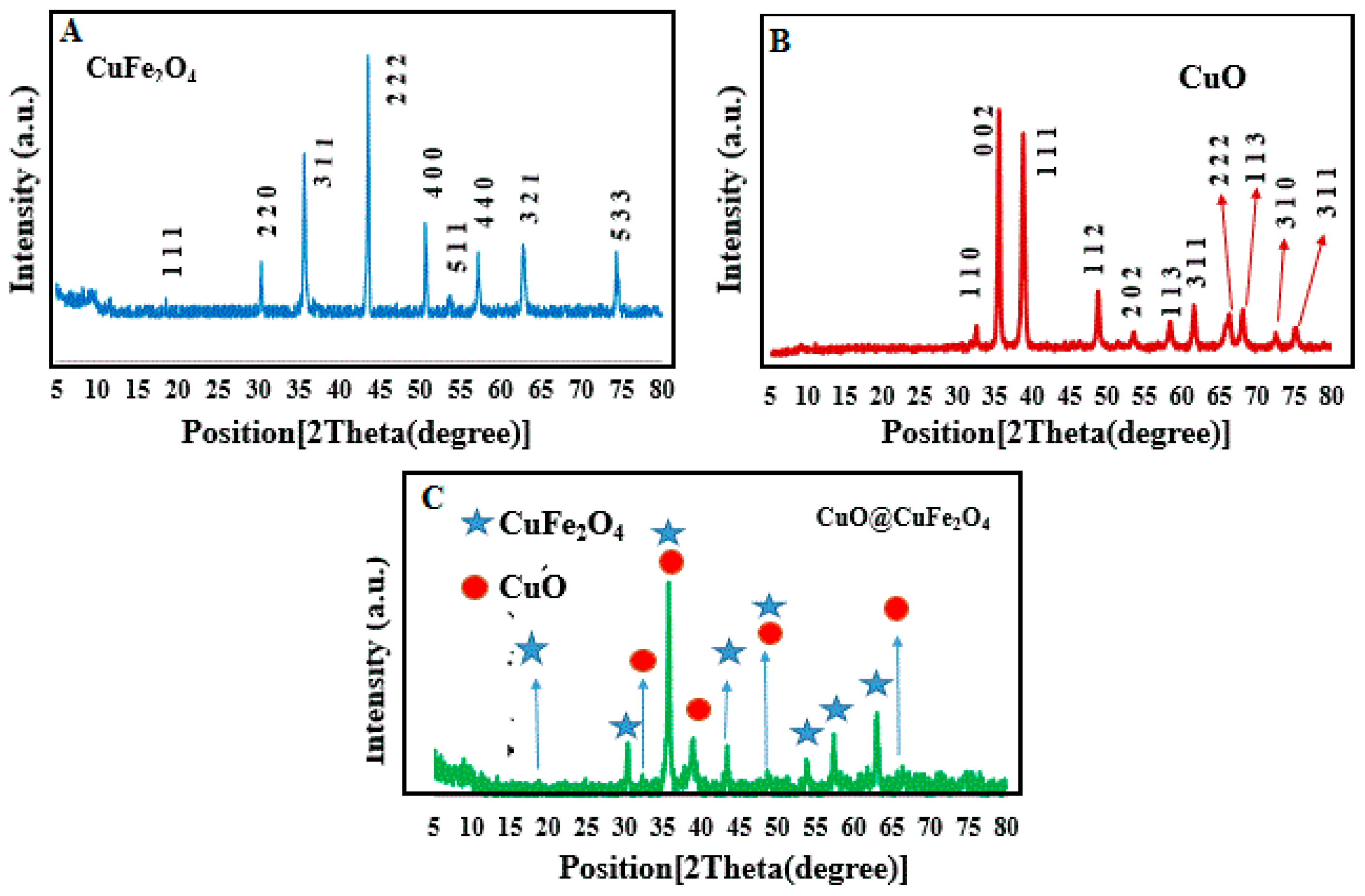
2.1. XRD Patterns
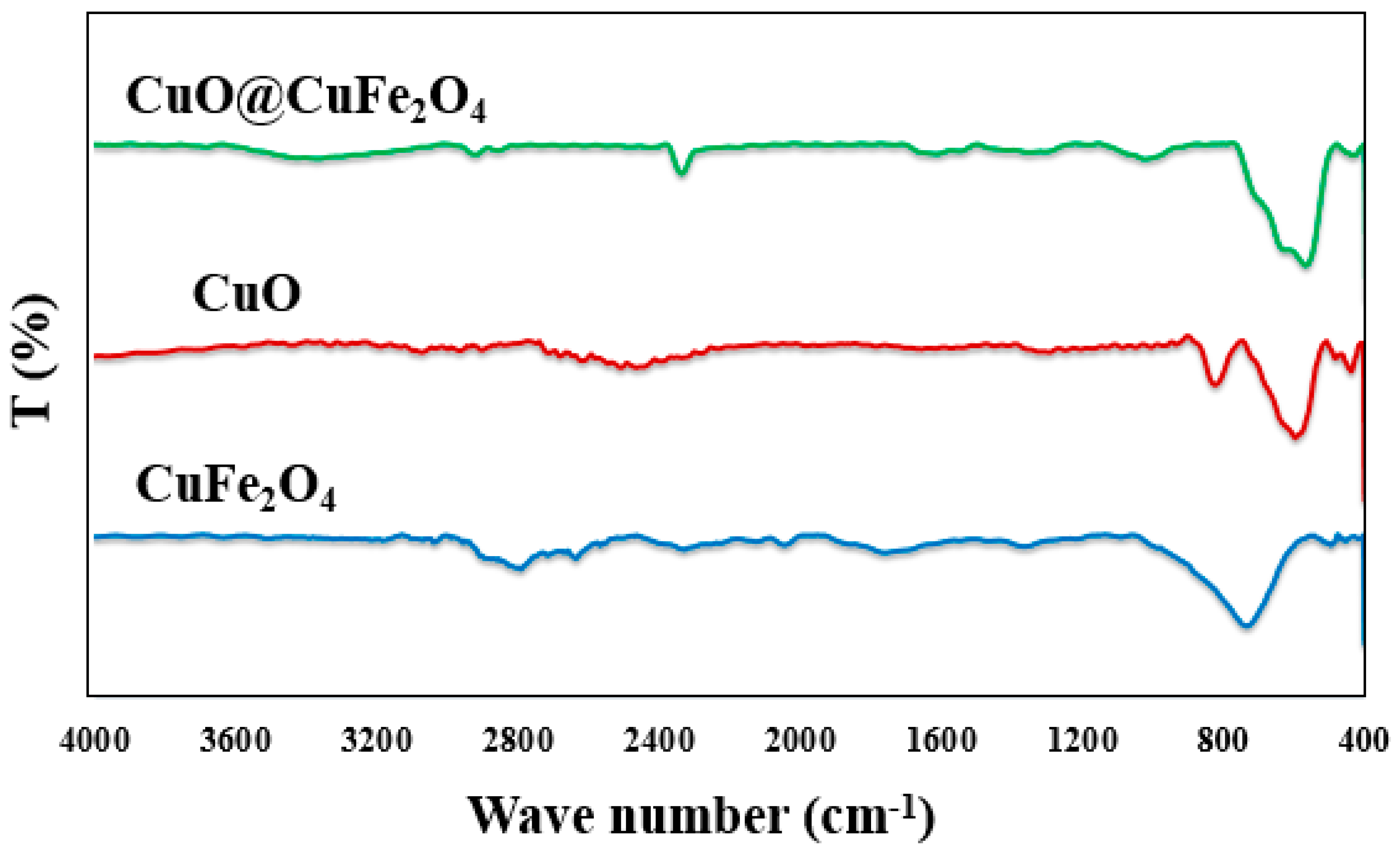
2.2. FT-IR Study
2.3. Morphological Analysis of CuFe2O4, CuO and Their Composite
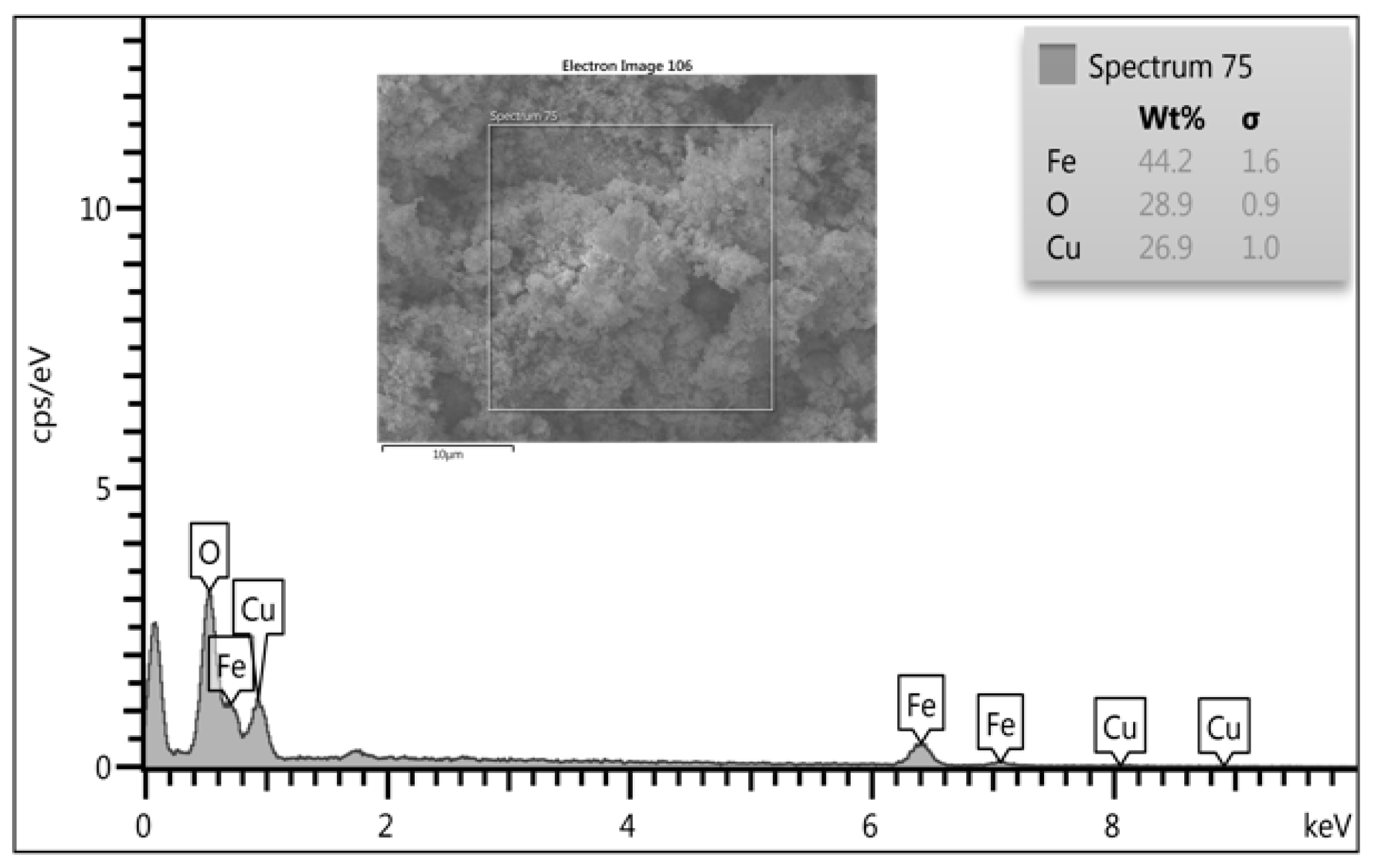
2.4. Elemental Analysis
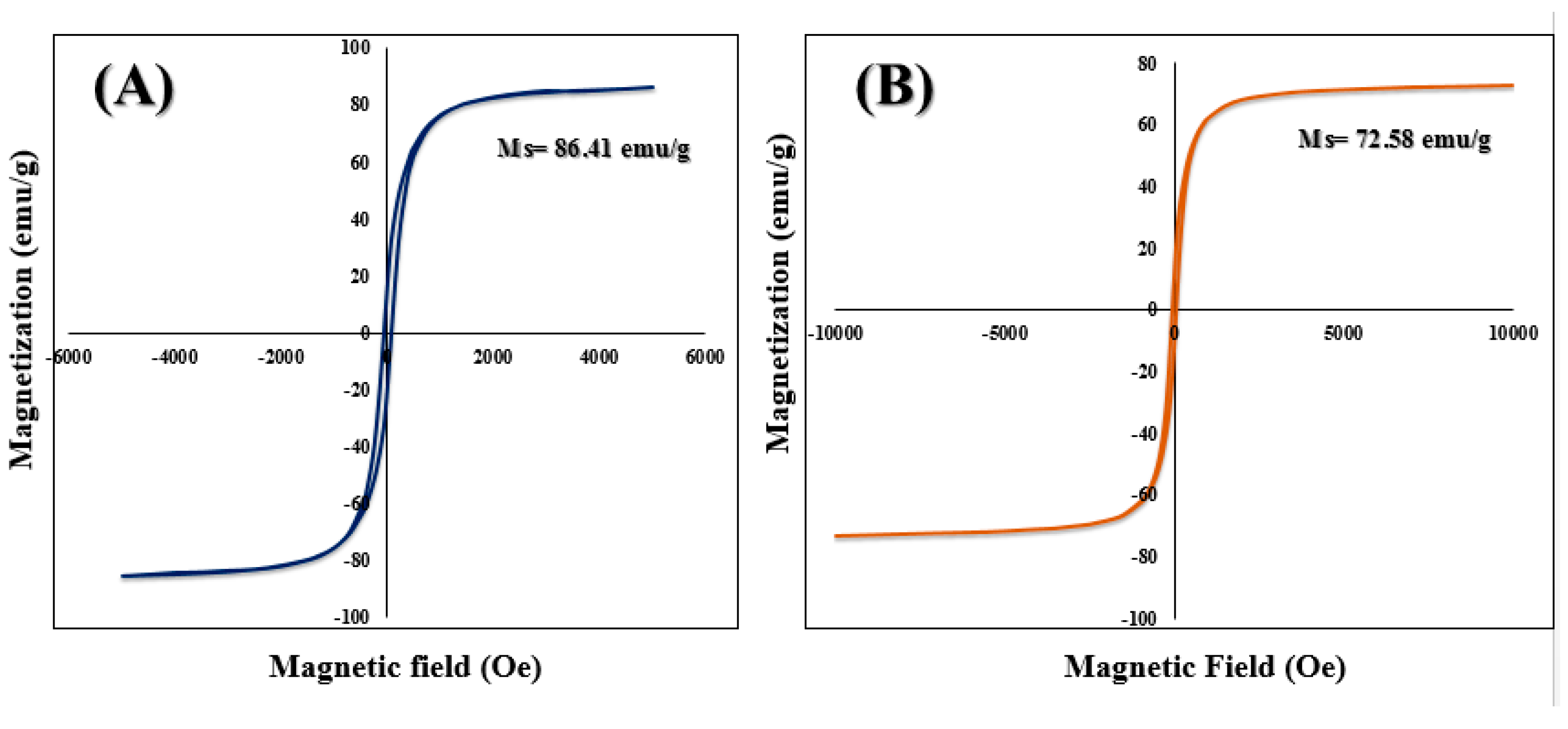
2.5. VSM Analysis
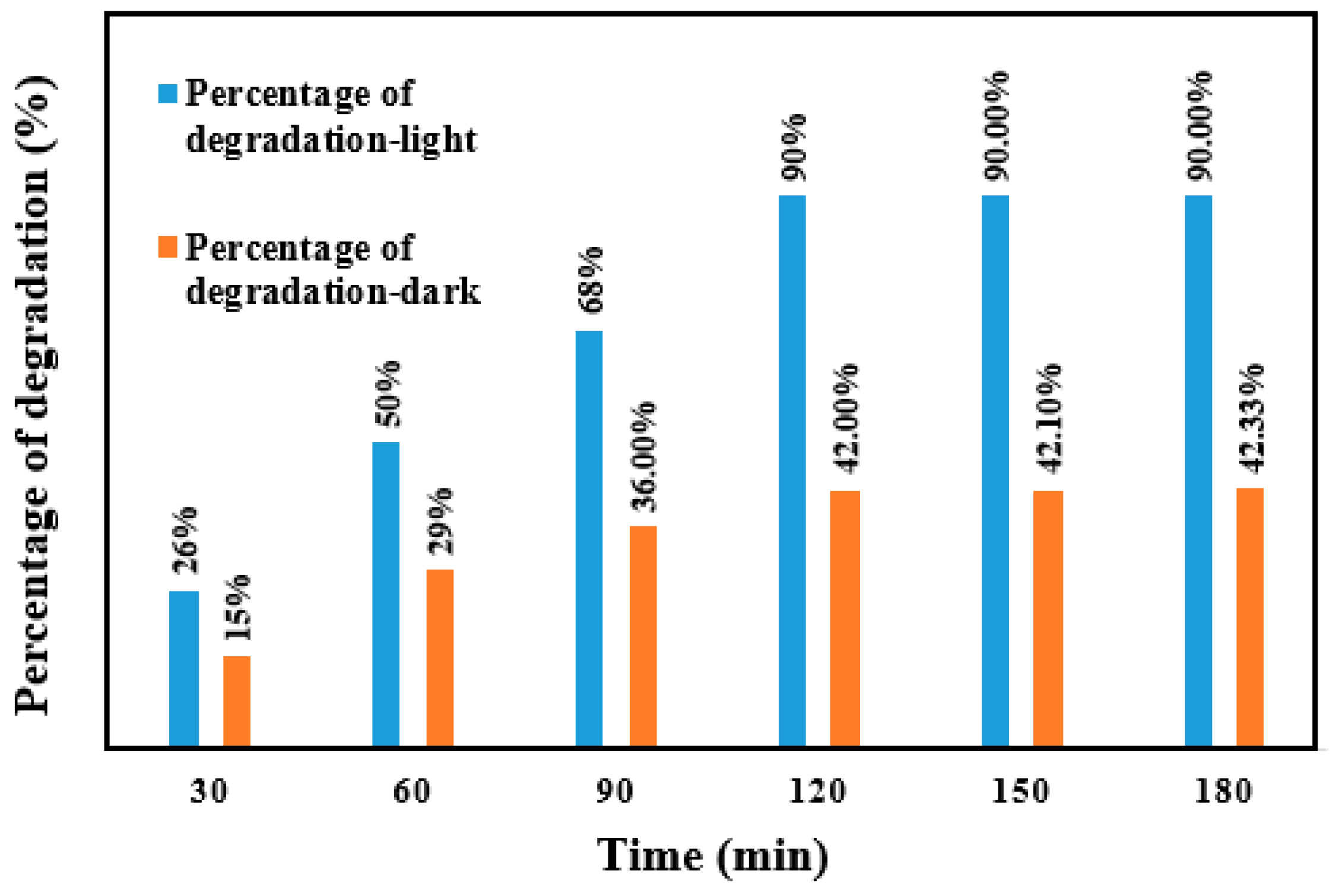
2.6. Photocatalytic Activity
3. Materials and Methods
3.1. Preparation of the CuO@CuFe2O4 Nanocomposite
3.2. Photocatalytic Experiments
4. Conclusions
References
- Greene, D.; Serrano-Garcia, R.; Govan, J.; Gun’ko, Y. Synthesis characterization and photocatalytic studies of cobalt ferrite-silica-titania nanocomposites. Nanomaterials 2014, 4, 331–343. [Google Scholar] [CrossRef]
- Comini, E.; Sberveglieri, G.; Ferroni, M.; Guidi, V.; Martinelli, G. Response to ethanol of thin films based on Mo and Ti oxides deposited by sputtering. Sens. Actuators B Chem. 2003, 93, 409–415. [Google Scholar] [CrossRef]
- Kasemr, K.K.; Rameyl, H.; Ahmed, V. Photo-Electrochemical Studies on TiO2-Doped Ce (III/IV) Oxides Nanoparticles in Aqueous Electrolytes. Mater. Sci. Appl. 2013, 4, 637. [Google Scholar] [CrossRef][Green Version]
- Carnes, C.L.; Klabunde, K.J. Unique chemical reactivities of nanocrystalline metal oxides toward hydrogen sulfide. Chem. Mater. 2002, 14, 1806–1811. [Google Scholar] [CrossRef]
- Rahimi, R.; Zargari, S.; Yousefi, A.; Berijani, M.Y.; Ghaffarinejad, A.; Morsali, A. Visible light photocatalytic disinfection of E. coli with TiO2-graphene nanocomposite sensitized with tetrakis (4-carboxyphenyl) porphyrin. Appl. Surf. Sci. 2015, 355, 1098–1106. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. Snapshots of a working catalyst: Possibilities and limitations of in situ spectroscopy in the field of heterogeneous catalysis. Chem. Commun. 2002, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Ren, T.Z.; Ma, T.Y.; Yuan, Z.Y. Hierarchical structures from inorganic nanocrystal self-assembly for photoenergy utilization. Int. J. Photoenergy 2014. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Goya, G.F.; Rechenberg, H.R. Magnetic properties of nanostructured CuFe2O4. J. Phys. Condensed Matter 1999, 11, 4063. [Google Scholar] [CrossRef]
- Liu, X.Q.; Tao, S.W.; Shen, Y.S. Preparation and characterization of nanocrystalline α-Fe2O3 by a sol-gel process. Sens. Actuators B Chem. 1997, 40, 161–165. [Google Scholar] [CrossRef]
- Pandya, P.B.; Joshi, H.H.; Kulkarni, R.G. Magnetic and structural properties of CuFe2O4 prepared by the co-precipitation method. J. Mater. Sci. Lett. 1991, 10, 474–476. [Google Scholar] [CrossRef]
- Verma, A.; Jaihindh, D.P.; Fu, Y.P. Photocatalytic 4-nitrophenol degradation and oxygen evolution reaction in CuO/gC3N4 composites prepared by deep eutectic solvent-assisted chlorine doping. Dalton Trans. 2019. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massoud-Sharifi, A.; Kara, G.K.; Rabbani, M. CuFe2O4@CuO: A Magnetic Composite Synthesized by Ultrasound Irradiation and Degradation of Methylene Blue on Its Surface in the Presence of Sunlight. Proceedings 2020, 48, 17. https://doi.org/10.3390/ECWS-4-06438
Massoud-Sharifi A, Kara GK, Rabbani M. CuFe2O4@CuO: A Magnetic Composite Synthesized by Ultrasound Irradiation and Degradation of Methylene Blue on Its Surface in the Presence of Sunlight. Proceedings. 2020; 48(1):17. https://doi.org/10.3390/ECWS-4-06438
Chicago/Turabian StyleMassoud-Sharifi, Ahmad, Gheffar K. Kara, and Mahboubeh Rabbani. 2020. "CuFe2O4@CuO: A Magnetic Composite Synthesized by Ultrasound Irradiation and Degradation of Methylene Blue on Its Surface in the Presence of Sunlight" Proceedings 48, no. 1: 17. https://doi.org/10.3390/ECWS-4-06438
APA StyleMassoud-Sharifi, A., Kara, G. K., & Rabbani, M. (2020). CuFe2O4@CuO: A Magnetic Composite Synthesized by Ultrasound Irradiation and Degradation of Methylene Blue on Its Surface in the Presence of Sunlight. Proceedings, 48(1), 17. https://doi.org/10.3390/ECWS-4-06438




