Applying Benzene Tetracarboxylic Acid as a Linker in the Synthesis a Porous Ba (II)-Based MOF by Ultrasonic Method †
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Instrumentation
2.2. Preparation of MOF
3. Results and Discussion
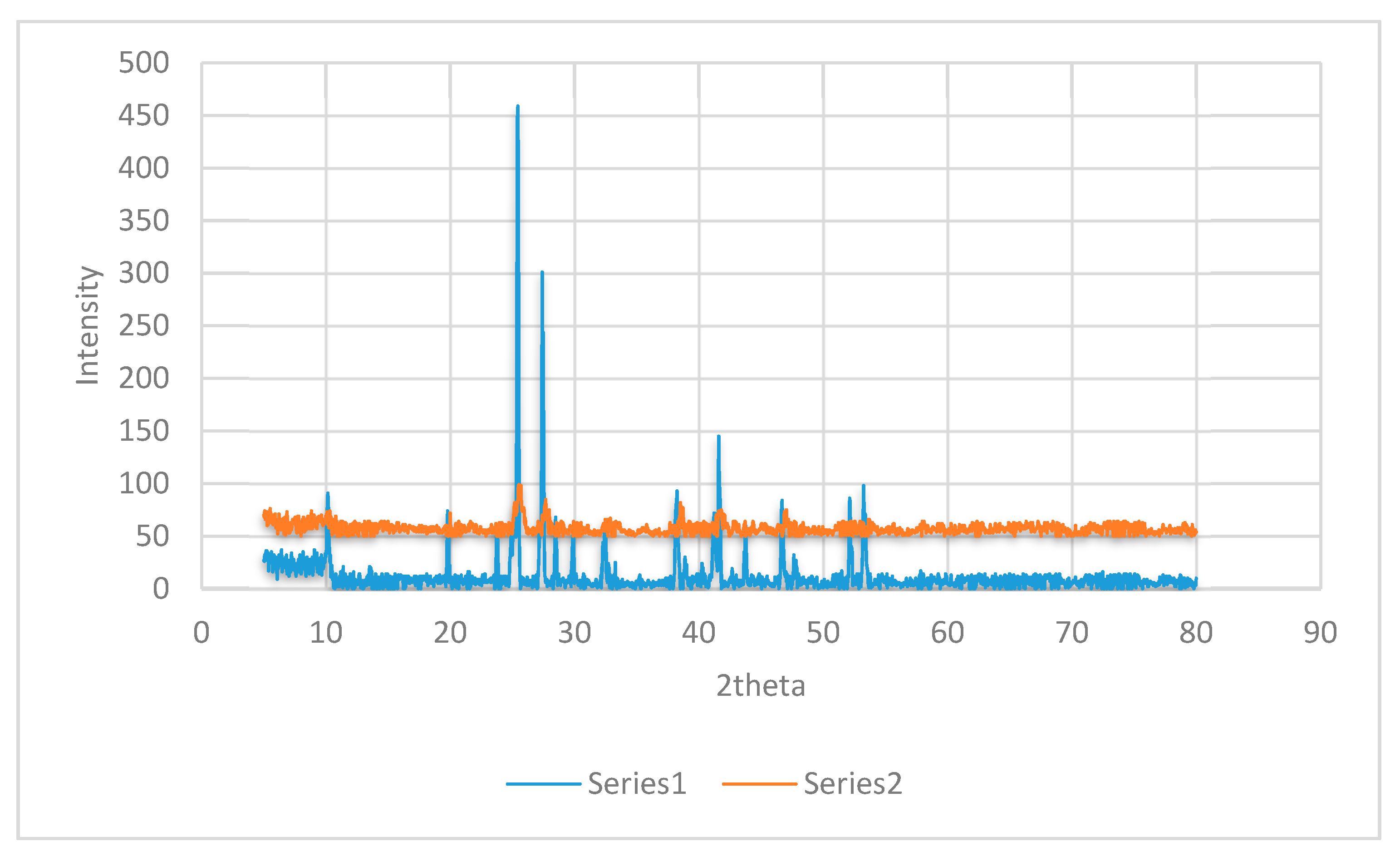
3.1. X-ray Diffraction
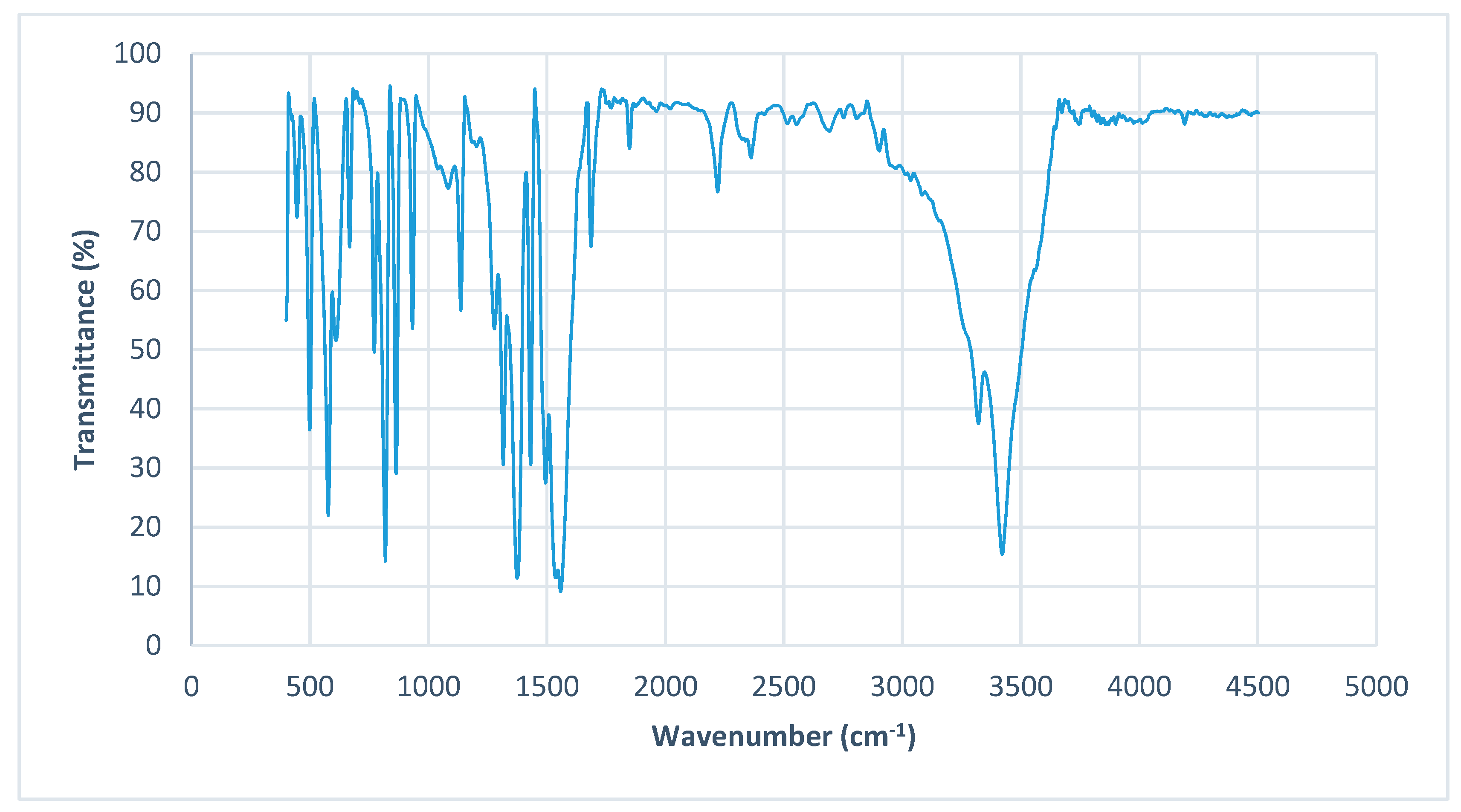
3.2. FTIR Spectra
3.3. SEM Analysis
4. Conclusions
References
- Rouhani, F.; Morsali, A. Frontispiece: Goal-Directed Design of Metal–Organic Frameworks for HgII and PbII Adsorption from Aqueous Solutions. Chem. A Eur. J. Chem. 2018, 24, 17170–17179. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, L.; Zhang, Z.; Sun, J.; Zhang, Y.; Jiang, J.Z. Synthesis, crystal structures, and fluorescence properties of porphyrin alkaline earth MOFs. Inorg. Chem. Commun. 2018, 95, 36–39. [Google Scholar] [CrossRef]
- Cao, K.L.; Xia, Y.; Wang, G.X.; Feng, Y.L. A robust luminescent Ba (II) metal–organic framework based on pyridine carboxylate ligand for sensing of small molecules. Inorg. Chem. Commun. 2015, 53, 42–45. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zheng, H.Q.; Zheng, H.Y.; Lin, L.P.; Xin, Q.; Cao, R. Efficient Capture and Effective Sensing of Cr2O72- from Water Using a Zirconium Metal-Organic Framework. Inorg. Chem. 2019, 56, 14178–14188. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, E.; Masoomi, M.Y.; Yamini, Y.; Morsali, A. Application of Mechanosynthesized Azine-Decorated Zinc(II) Metal–Organic Frameworks for Highly Efficient Removal and Extraction of Some Heavy-Metal Ions from Aqueous Samples: A Comparative Study. Inorg. Chem. 2015, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dung, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; et al. Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv. 2015, 5, 5261–5268. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, G.; Chen, H.; Hu, X.; Niu, Z.; Ma, S. Functionalized metal–organic framework as a new platform for efficient and selective removal of cadmium (II) from aqueous solution. J. Mater. Chem. A 2015, 3, 15292–15298. [Google Scholar] [CrossRef]
- Halake, S.; Ok, K.M. differential gas adsorption, high thermal stability, and reversible coordination of two new barium-organic frameworks, Ba(SBA)(DMF)4 and Ba2(BTEC)(H2O). J. Solid State Chem. 2015, 231, 132–137. [Google Scholar] [CrossRef]
- Balendra, A.; Ramanan, A. Structural diversity of alkaline-earth 2,5-thiophenedicarboxylates. J. Mol. Struct. 2017, 1131, 171–180. [Google Scholar] [CrossRef]
- Du, S.; Ji, C.; Xin, X.; Zhuang, M.; Yu, X.; Lu, J.; Lu, Y.; Sun, D. Syntheses, structures and characteristics of four alkaline-earth metal-organic frameworks (MOFs) based on benzene-1,2,4,5-tetracarboxylicacid and its derivative ligand. J. Mol. Struct. 2017, 1130, 565–572. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nezhad, T.R.M.; Manteghi, F. Applying Benzene Tetracarboxylic Acid as a Linker in the Synthesis a Porous Ba (II)-Based MOF by Ultrasonic Method. Proceedings 2019, 41, 61. https://doi.org/10.3390/ecsoc-23-06620
Nezhad TRM, Manteghi F. Applying Benzene Tetracarboxylic Acid as a Linker in the Synthesis a Porous Ba (II)-Based MOF by Ultrasonic Method. Proceedings. 2019; 41(1):61. https://doi.org/10.3390/ecsoc-23-06620
Chicago/Turabian StyleNezhad, Targol Rahimi Masale, and Faranak Manteghi. 2019. "Applying Benzene Tetracarboxylic Acid as a Linker in the Synthesis a Porous Ba (II)-Based MOF by Ultrasonic Method" Proceedings 41, no. 1: 61. https://doi.org/10.3390/ecsoc-23-06620
APA StyleNezhad, T. R. M., & Manteghi, F. (2019). Applying Benzene Tetracarboxylic Acid as a Linker in the Synthesis a Porous Ba (II)-Based MOF by Ultrasonic Method. Proceedings, 41(1), 61. https://doi.org/10.3390/ecsoc-23-06620



