Multicomponet Synthesis of Pyrrolo [3,4-a] Carbazole-1,3-Diones †
Abstract
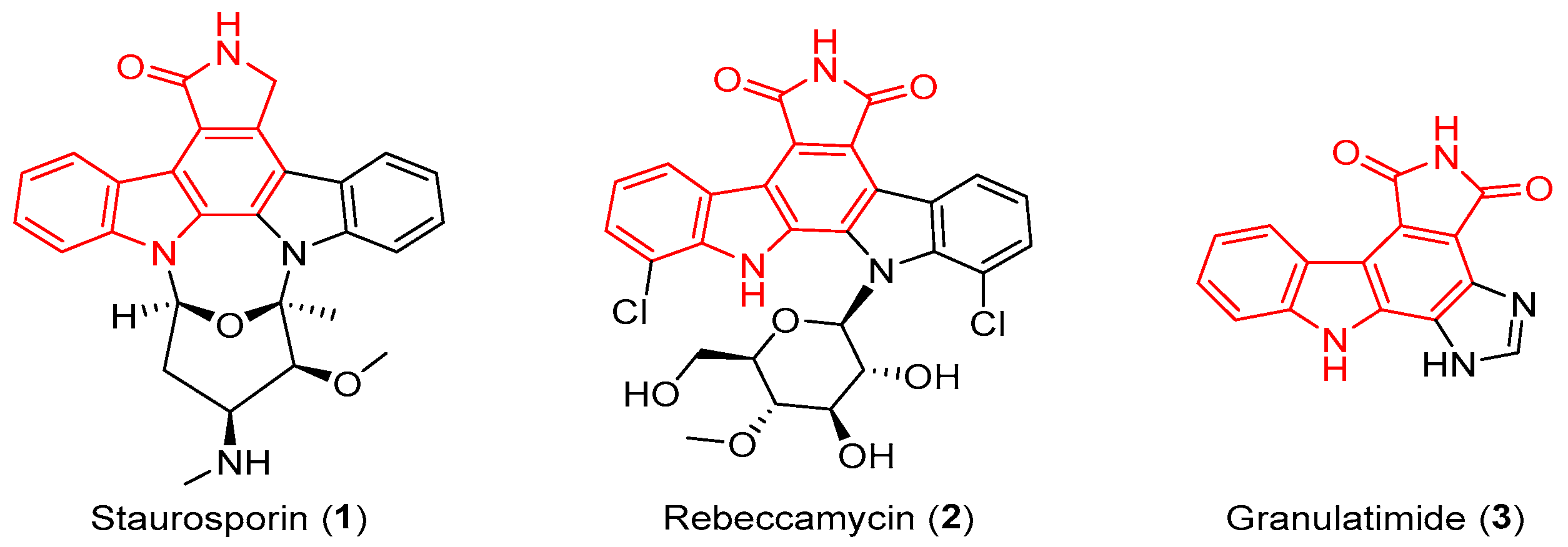
:1. Introduction
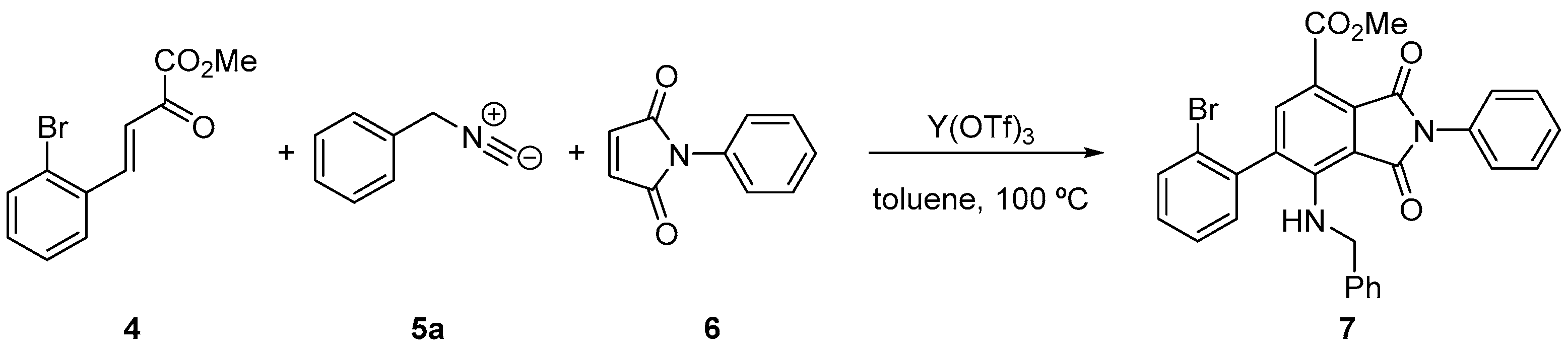
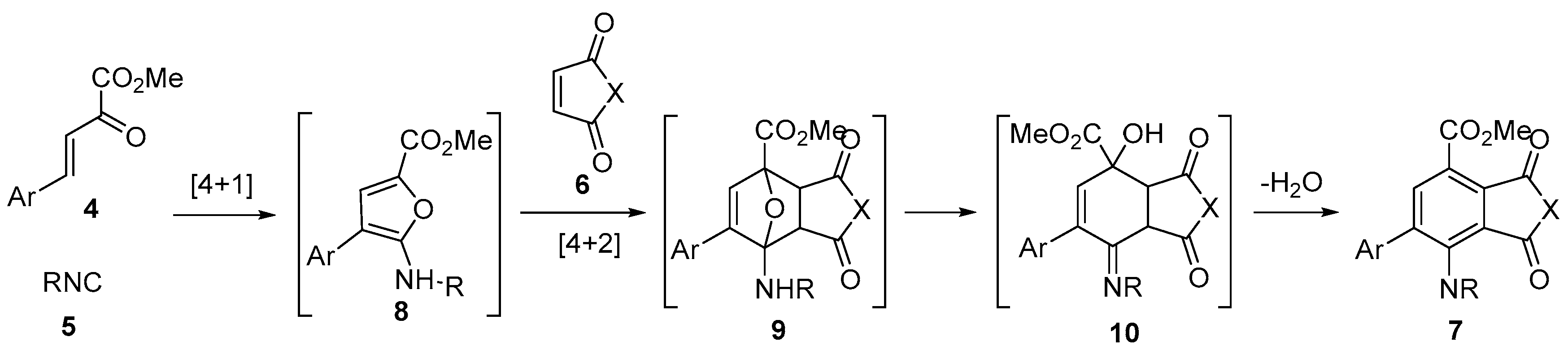
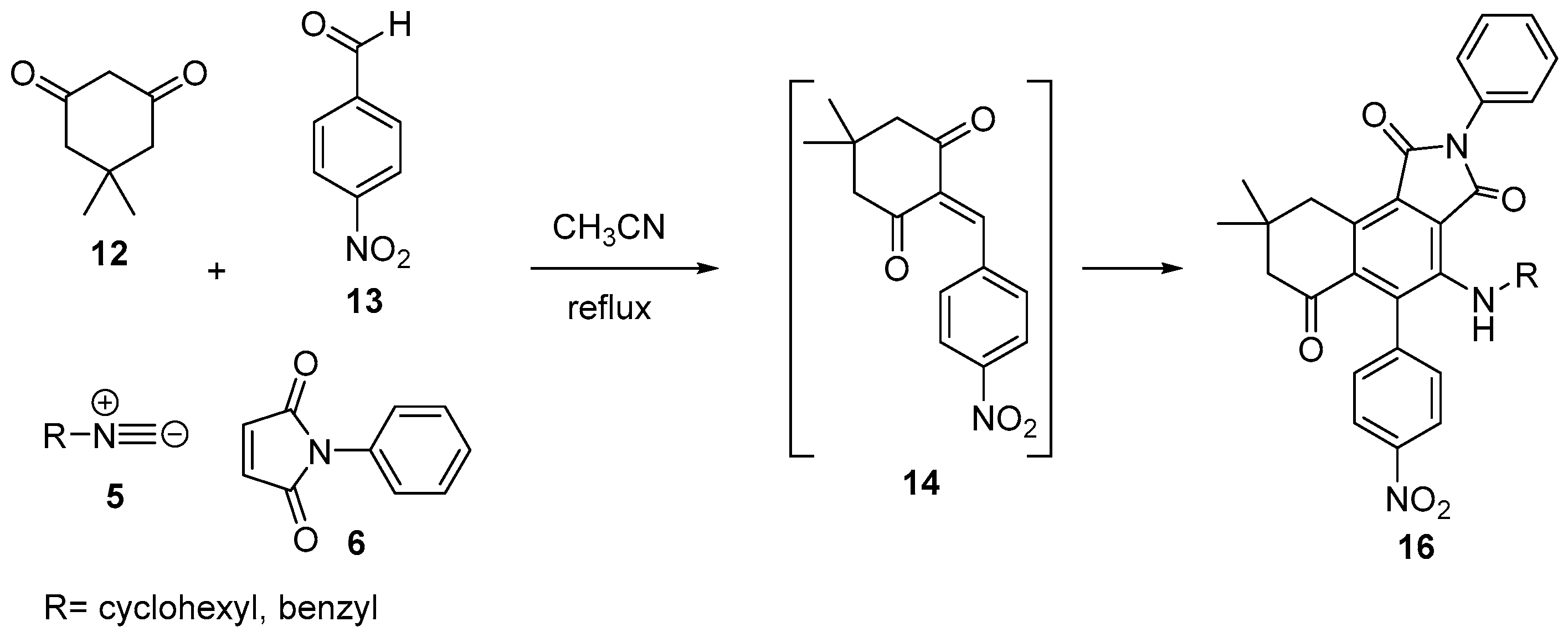
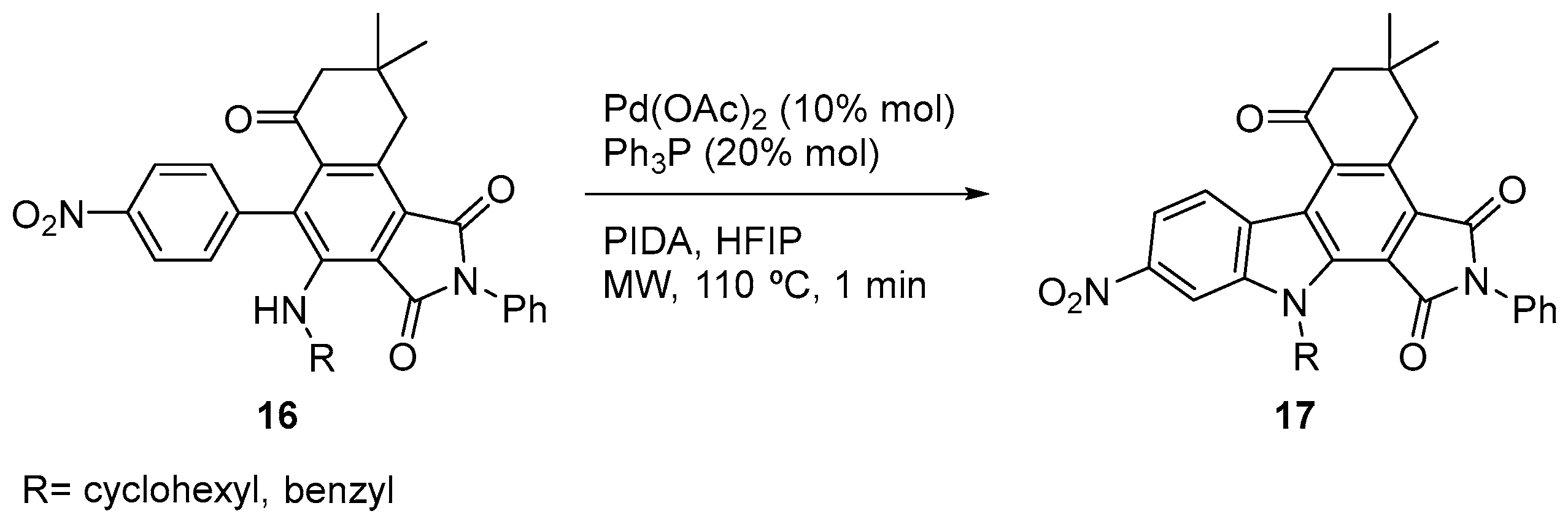
2. Results and Discussion
3. Conclusions
4. Experimental Section
Acknowledgments
References
- Greger, H. Phytocarbazoles: Alkaloids with great structural diversity and pronounced biological activities. Phytochem. Rev. 2017, 16, 1095–1153. [Google Scholar] [CrossRef]
- Schmidt, A.W.; Reddy, K.R.; Knolker, H.J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012, 112, 3193–3328. [Google Scholar] [CrossRef]
- Issa, S.; Prandina, A.; Bedel, N.; Rongved, P.; Yous, S.; Le Borgne, M.; Bouaziz, Z. Carbazole scaffolds in cancer therapy: A review from 2012 to 2018. J. Enzym. Inhib. Med. Chem. 2019, 34, 1321–1346. [Google Scholar] [CrossRef]
- Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M.V.; Bruno, R.; Aquaro, S.; Sinicropi, M.S. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules 2019, 24, 1912–1936. [Google Scholar] [CrossRef]
- Sherer, C.; Snape, T.J. Heterocyclic scaffolds as promising anticancer agents against tumours of the central nervous system: Exploring the scope of indole and carbazole derivatives. Eur. J. Med. Chem. 2015, 97, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Głuszyńska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef]
- Krucaite, G.; Grigalevicius, S. A review on low-molar-mass carbazole- based derivatives for organic light emitting diodes. Synth. Met. 2019, 247, 90–108. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, C.; Qin, J. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, G.; Sivakumar, E.K.T.; Ganesamoorthy, R.; Thangamuthu, R.; Sakthivel, P. Review of carbazole based conjugated molecules for highly efficient organic solar cell application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Li, J.; Grimsdale, A.C. Carbazole-based polymers for organic photovoltaic devices. Chem. Soc. Rev. 2010, 39, 2399–2410. [Google Scholar] [CrossRef]
- Janosik, T.; Rannug, A.; Rannug, U.; Wahlström, N.; Slätt, J.; Bergman, J. Chemistry and Properties of Indolocarbazoles. Chem. Rev. 2018, 118, 9058–9128. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Mendez, C.; Salas, J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006, 23, 1007–1045. [Google Scholar] [CrossRef]
- Conchon, E.; Anizon, F.; Aboab, B.; Golsteyn, R.M.; Léonce, S.; Pfeiffer, B.; Prudhomme, M. Synthesis, in vitro antiproliferative activities, and Chk1 inhibitory properties of pyrrolo [3,4-a] carbazole-1,3-diones, pyrrolo[3,4-c]carbazole-1,3-diones, and 2-aminopyridazino[3,4-a]pyrrolo[3,4-c]carbazole-1,3,4,7-tetraone. Eur. J. Med. Chem. 2008, 43, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.T.; Facompré, M.; Da Costa, H.; Routier, S.; Mérour, J.Y.; Colson, P.; Houssier, C.; Bailly, C. Synthesis, cytotoxicity, DNA interaction and topoisomerase II inhibition properties of tetrahydropyrrolo [3,4-a] carbazole-1,3-dione and tetrahydropyrido-[3,2-b]pyrrolo[3,4-g]indole-1,3-dione derivatives. Biorg. Med. Chem. 2001, 9, 1533–1541. [Google Scholar] [CrossRef]
- Giraud, F.; Pereira, E.; Anizon, F.; Moreau, P. Synthesis and applications of dihydropyrrolocarbazoles. Eur. J. Org. Chem. 2019, 2019, 5025–5042. [Google Scholar] [CrossRef]
- Pindur, U.; Otto, C. A new access to 2′-amino-substituted vinylindoles as donor-activated heterocyclic dienes and their first diels-alder reactions. Tetrahedron 1992, 48, 3515–3526. [Google Scholar] [CrossRef]
- Alonso, M.Á.; López-Alvarado, P.; Avendaño, C.; Menéndez, J.C. Regioselective Diels–Alder reactions of 3-indolylquinones. Tetrahedron 2003, 59, 2821–2830. [Google Scholar] [CrossRef]
- Neo, A.G.; Bornadiego, A.; Díaz, J.; Marcaccini, S.; Marcos, C.F. Elusive 2-aminofuran Diels-Alder substrates for a straightforward synthesis of polysubstituted anilines. Org. Biomol. Chem. 2013, 11, 6546–6555. [Google Scholar] [CrossRef]
- Bornadiego, A.; Díaz, J.; Marcos, C.F. Tandem synthesis of polycyclic isoindoles. J. Org. Chem. 2019, 84, 7426–7433. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Wadhwa, P.; Bagchi, S.; Sharma, A. Isocyanide based [4+1] cycloaddition reactions: An indispensable tool in multi-component reactions (MCRs). Chem. Commun. 2016, 52, 6958–6976. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Menon, R.S.; Vinod, A.U.; Viji, S. A facile three-component reaction involving [4+1] cycloaddition leading to furan annulated heterocycles. Tetrahedron Lett. 2002, 43, 2293–2295. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, S.Y.; Youn, S.W. Pd-catalyzed sequential C-C and C-N bond formations for the synthesis of N-heterocycles: Exploiting protecting group-directed C-H activation under modified reaction conditions. Chem. Asian J. 2011, 6, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Antonchick, A.P.; Samanta, R.; Kulikov, K.; Lategahn, J. Organocatalytic, oxidative, intramolecular C-H bond amination and metal-free cross-amination of unactivated arenes at ambient temperature. Angew. Chem. Int. Ed. 2011, 50, 8605–8608. [Google Scholar] [CrossRef] [PubMed]
- Jordan-Hore, J.A.; Johansson, C.C.; Beck, E.M.; Gaunt, M.J. Oxidative Pd (II)-catalyzed C-H bond amination to carbazole at ambient temperature. J. Am. Chem. Soc. 2008, 130, 16184–16186. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.C.; Zheng, N.; Buchwald, S.L. Combined C-H functionalization/C-N bond formation route to carbazoles. J. Am. Chem. Soc. 2005, 127, 14560–14561. [Google Scholar] [CrossRef] [PubMed]
- Conchon, E.; Anizon, F.; Aboab, B.; Prudhomme, M. Synthesis and Biological Activities of New Checkpoint Kinase 1 Inhibitors Structurally Related to Granulatimide. J. Med. Chem. 2007, 50, 4669–4680. [Google Scholar] [CrossRef]
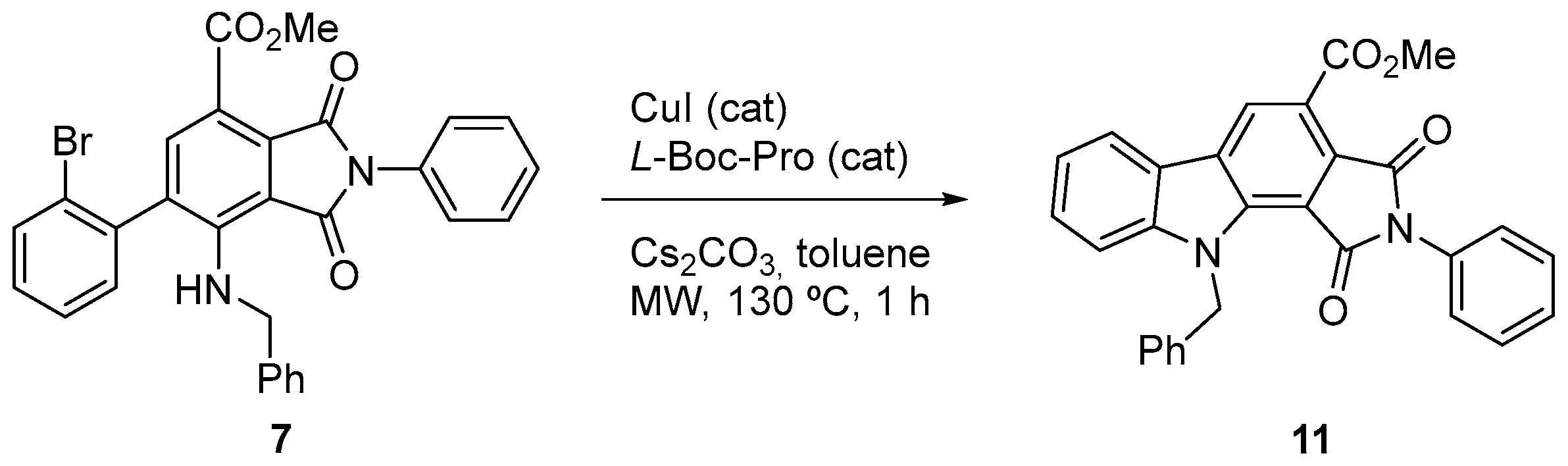
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bornadiego, A.; Neo, A.G.; Díaz, J.; Marcos, C.F. Multicomponet Synthesis of Pyrrolo [3,4-a] Carbazole-1,3-Diones. Proceedings 2019, 41, 50. https://doi.org/10.3390/ecsoc-23-06525
Bornadiego A, Neo AG, Díaz J, Marcos CF. Multicomponet Synthesis of Pyrrolo [3,4-a] Carbazole-1,3-Diones. Proceedings. 2019; 41(1):50. https://doi.org/10.3390/ecsoc-23-06525
Chicago/Turabian StyleBornadiego, Ana, Ana G. Neo, Jesús Díaz, and Carlos F. Marcos. 2019. "Multicomponet Synthesis of Pyrrolo [3,4-a] Carbazole-1,3-Diones" Proceedings 41, no. 1: 50. https://doi.org/10.3390/ecsoc-23-06525
APA StyleBornadiego, A., Neo, A. G., Díaz, J., & Marcos, C. F. (2019). Multicomponet Synthesis of Pyrrolo [3,4-a] Carbazole-1,3-Diones. Proceedings, 41(1), 50. https://doi.org/10.3390/ecsoc-23-06525





