Abstract
Widespread secondary plant metabolites (betulinic, ursolic, and oleanolic acids) are promising scaffolds for the discovery of new drugs. Among the many semi-synthetic derivatives of lupane triterpenoids known today, the anticancer agent C-28 ester of betulinic acid with tris(hydroxymethyl)aminomethane (NVX-207) has been actively studied. This betulinic acid derivative, which has shown significant antitumor activity in vitro and in vivo against various malignant tumors, is a candidate drug. It is known that modification of the structure of the triterpenoid skeleton can lead to significant changes in biological properties. In this regard, we have synthesized C-28 esters of ursolic and oleanolic acids and C20-29 hydrogenated betulinic acid bearing tris(hydroxymethyl)aminomethane (TRIS), and transformed them into guanidinium salts by guanylation of the primary amino group in a branched ester fragment under the action of 1H-pyrazole-1-carboxamidine hydrochloride. The obtained compounds were tested in in vitro experiments on three human cancer cell lines. The presence of the TRIS-fragment in the triterpenoid conjugates markedly enhanced the cytotoxic action as compared to the parent compounds, dihydrobetulinic, ursolic, and oleanolic acids (IC50 values 2.8–7.6 μM for Jurkat cells and 1.1–6.8 μM for U937 cells), while the correlation between the cytotoxic activity and the chemical structure of the triterpenoid skeleton was not observed. Extended biological testing of these triterpenoids by using flow cytometry analysis showed that antitumor activity of compounds is caused by apoptotic processes and induction of cell cycle arrest in the S-phase. New triterpenoids were also tested for their antimicrobial activity against the growth of four bacterial strains (Escherichia coli, Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus (MRSA)) and two fungal strains (Candida albicans and Cryptococcus neoformans). The TRIS-dihydrobetulinic acid conjugate and its guanidinium derivative did not exhibit antimicrobial properties. The corresponding ursane and oleane-skeleton pentacyclic tritrerpenoids showed good bacteriostatic activity against methicillin-resistant S. aureus (MICs 4 and 32 μg/mL) and excellent antifungal effect against Cryptococcus neoformans and Candida albicans (MICs 4 and 0.25 μg/mL).
1. Introduction
Among natural compounds of plant origin, which are considered as the richest sources of leading structures for drug development, a significant place is occupied by pentacyclic triterpenoid acids of the lupane, ursane, and oleanane group (Figure 1) [1,2]. These compounds are produced as a result of squalene cyclization and are present everywhere in various parts of plants—in the bark, in the wax coating of the leaves, or in the peel of the fruit.
Figure 1.
Dihydro betulinic, ursolic, and oleanolic acids.
Triterpenoid acids exhibit a wide variety of biological activities, which successfully combine with low systemic toxicity. The greatest interest in these compounds is due to their antitumor (anticancer), antiviral, antibacterial, and antiparasitic properties [3,4,5,6]. However, the relatively low potential biological activity of native triterpenoid acids, their poor solubility in aqueous medium, and insufficient bioavailability cause serious problems for promoting these compounds to clinical practice.
Natural pentacyclic triterpenoid acids have high synthetic potential due to the easily transformed functional groups (3-OH, 28-COOH) present in their molecules. During the last decade numerous derivatives of pentacyclic triterpenoids have been produced by modifying functional groups at the C-3 and C-28 atoms of triterpene core, which in some cases were more efficient in their biology action than their prototypes [3]. One of the most well-known compounds of this type is the C-28 ester of betulinic acid bearing the tris(hydroxymethyl)aminomethane fragment (NVX-207). (Figure 2).
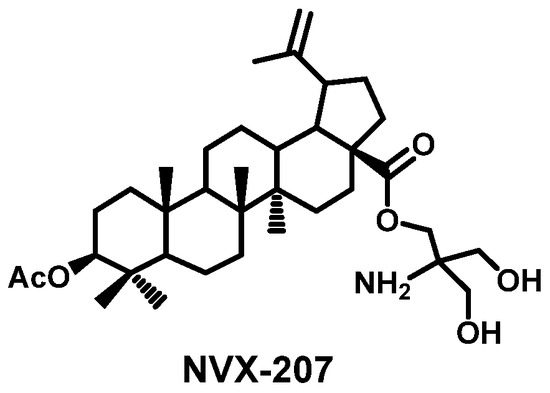
Figure 2.
Structure of compound NVX-207.
This promising anticancer agent has shown higher cytotoxicity as compared to betulinic acid against diverse tumor cell lines in humans and dogs. In addition, the use of NVX-207 in the phase I/II clinical trials in large animals led to the complete remission of resistant tumors [7,8,9,10]. Small structural alterations of this lupane triterpenoid significantly influenced its anticancer activity.
In this connection, we have synthesized new structural analogues of NVX-207, namely, the C-28 esters of ursolic and oleanolic acids, as well as the ester of C20-29 hydrogenated betulinic acid having tris(hydroxymethyl)aminomethane moiety (TRIS). The resulting compounds were converted into guanidinium salts by guanilation of primary amine groups in the branched-chain ester fragment.
The guanidine group is a common key unit in various natural and synthetic compounds demonstrating antimicrobial, antiviral, and antitumor activities [11]. Furthermore, because of high basicity (pKa 13.5), the guanidinium group is important for selective delivery of cytotoxic molecules to tumor cells [12].
In this paper, we discuss the effect of these structural changes on the antitumor and antimicrobial activity.
2. Experimental
2.1. Chemistry
All reagents and solvents were of the purest grade available, and were generally used without further treatment. The starting compounds (ursolic, oleanolic acids) and reagents: oxalyl chloride, dimethylaminopyridine (DMAP), tris(hydroxymethyl)aminomethane, triethylamine (Et3N), N,N-diisopropylethylamine (DIPEA), and 1H-pyrazole-1-carboxamidine hydrochloride were purchased from Acros Organics (Geel, Belgium). Dihydrobetulinic acid was obtained from betulin according to the known procedure [13]. Acetates of oleanolic, ursolic, and dihydrobetulinic acids were synthesized according to the typical procedures. Synthesis and (1H, 13C) NMR spectra of compounds NVX-207, 2, 5, 8, 5a, and 8a are in the works reported previously [7,14].
2.1.1. General Procedure for the Synthesis of Amines 2, 5, 8 and Amides 5a, 8a
Oxalyl chloride (0.13 mL, 1.5 mmol) was added with stirring to a solution of compounds 1, 4, or 7 (0.5 mmol) in dry CH2Cl2 (5 mL) precooled to 0 °C, and stirring of the reaction mixture was continued at room temperature for 2 h. Then the solvents with excess oxalyl chloride were removed under vacuum and the acid chlorides of 1, 4, and 7 (1 mmol), dissolved in a mixture of pyridine (4 mL), CH2Cl2 (1 mL), and DMAP (0.09 g, 0.7 mmol), were added. After complete dissolution of DMAP, a solution containing TRIS (tris(hydroxymethyl)aminomethane) (0.24 g, 2 mmol) in pyridine (0,5 mL) was added. The mixture was stirred for 10 h at room temperature and the solvent was removed rapidly under vacuum. The residue was chromatographed on silica gel, using CH2Cl2/MeOH 30:1→1:1, to obtain pure compounds 2, 5, 8, 5a, and 8a.
2.1.2. General Procedure for the Guanilation of Amines 2, 5, and 8
The amine (0.5 mmol) was dissolved in dry DMF (1 mL) and under vigorous stirring DIPEA (0.2 mL, 1.5 mmol) and 1H-pyrazole-1-carboxamidine hydrochloride (0.09 g, 0.6 mmol) were added. The mixture was stirred for 24 h (monitoring by TLC). The mixture is diluted with cold H2O and the precipitate which formed was filtered off and washed with water to obtain pure compounds 3, 6, and 9.
2.2. Biology
2.2.1. Anticancer Activity
Cell Culturing
Cells (Jurkat, K562, U937) were purchased from Russian Cell Culture Collection (Institute of Cytology of the Russian Academy of Sciences, Saint Petersburg, Russia) and cultured according to standard mammalian tissue culture protocols and sterile technique. All cell lines used in the study were tested and shown to be free of mycoplasma and viral contamination. Cells were maintained in RPMI 1640 Media (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 4 mM glutamine, 10% FBS (Sigma, Burlington, MA, USA), and 100 units/mL penicillin–streptomycin (Sigma Burlington, MA, USA). All types of cells were grown in an atmosphere of 5% CO2 at 37 °C. The cells were sub-cultured at 2-day intervals with a seeding density of 1 × 105 cells per 24-well plate in RPMI with 10% FBS.
Cytotoxicity Assay
Viability (Live/dead) assessment was performed by staining cells with 7-aminoactinomycin D (7-AAD) (Biolegend, San Diego, CA, USA). Cells were treated of test compounds with six different concentrations (1, 5, 10, 15, 30, and 60 μM). After treatment, cells were harvested, washed 1–2 times with phosphate-buffered saline (PBS), and centrifuged at 400× g for 5 min. Cell pellets were resuspended in 200 μL of flow cytometry staining buffer (PBS without Ca2+ and Mg2+, 2.5% FBS) and stained with 5 μL of 7-AAD staining solution for 15 min at room temperature in the dark. Samples were acquired on a NovoCyteTM 2000 FlowCytometry System (ACEA, San Diego, CA, USA) equipped with 488 nm argon laser. Detection of 7-AAD emission was collected through a 675/30 nm filter in FL4 channel.
Viability and Apoptosis
Apoptosis was determined by flow cytometric analysis of Annexin V and 7-aminoactinomycin D (7-AAD) staining. Briefly, 200 μL of Guava Nexin reagent (Millipore, Bedford, MA, USA) were added to 5 × 105 cells in 200 μL, and the cells were incubated with the reagent for 20 min at room temperature in the dark. The plates were treated with compound 2 and dihydrobetulinic acid at IC50 concentration (4 and 59 μM) for 24 h and 48 h. At the end of incubation, the cells were analyzed on NovoCyteTM 2000 FlowCytometry System (ACEA). Different states of cell death were defined as follows: normal cells are localized in the lower-left quadrant; early apoptotic cells are in the lower-right quadrant; late apoptotic cells and necrotic cells are in the upper-right quadrant; and necrotic cells are in the upper-left quadrant.
Cell Cycle Analysis
Cell cycle was analyzed using the method of propidium iodide staining. Briefly, cells were plated in 24-well round bottom plates at density 10 × 105 cells per well, centrifuged at 450× g for 5 min, and fixed with ice-cold 70% ethanol for 24 h at 0 °C. Cells were then washed with PBS and incubated with 250 μL of Guava Cell Cycle Reagent (Millipore, Burlington, MA, USA) for 30 min at room temperature in the dark. Samples were analyzed on NovoCyteTM 2000 FlowCytometry System (ACEA, San Diego, CA, USA).
2.2.2. Antimicrobial Activity
The minimum inhibitory concentrations (MICs) of compounds NVX-207, 2, 3, 5, 6, 8, and 9 were determined against Gram-negative bacterial strains Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Gram-positive bacteria Staphylococcus aureus methicillin-resistant (MRSA) strain types. Antifungal activity was determined against Candida albicans and Cryptococcus neoformans. The antimicrobial screening performed by CO-ADD (The Community for Antimicrobial Drug Discovery) was funded by the Wellcome Trust (UK) and The University of Queensland (Australia) [15].
Antibacterial Assays
All bacteria were cultured in cation-adjusted Mueller–Hinton broth (CAMHB) at 37 °C overnight. A sample of each culture was then diluted 40-fold in fresh broth and incubated at 37 °C for 1.5–3 h. The resultant mid-log phase cultures were diluted (CFU/mL measured by OD600), then added to each well of the compound-containing plates, giving a cell density of 5 × 105 CFU/mL and a total volume of 50 μL. All the plates were covered and incubated at 37 °C for 18 h without shaking. Inhibition of bacterial growth was determined measuring absorbance at 600 nm (OD600), using a Tecan M1000 Pro monochromator plate reader.
The percentage of growth inhibition was calculated for each well, using the negative control (media only) and positive control (bacteria without inhibitors) on the same plate as references. The MIC was determined as the lowest concentration, at which the growth was fully inhibited, defined by an inhibition ≥ 80%. In addition, the maximal percentage of growth inhibition is reported as DMax, indicating any compounds with partial activity.
Hits were classified by MIC ≤ 16 µg/mL or MIC ≤ 10 µM in either replicate (n = 2 on different plates).
Antifungal Assays
Fungi strains were cultured for 3 days on Yeast Extract-Peptone Dextrose (YPD) agar at 30 °C. A yeast suspension of 1 × 106 to 5 × 106 CFU/mL (as determined by OD530) was prepared from five colonies. The suspension was subsequently diluted and added to each well of the compound-containing plates giving a final cell density of fungi suspension of 2.5 × 103 CFU/mL and a total volume of 50 μL. All plates were covered and incubated at 35 °C for 36 h without shaking.
Growth inhibition of C. albicans was determined measuring absorbance at 630 nm (OD630), while the growth inhibition of C. neoformans was determined measuring the difference in absorbance between 600 and 570 nm (OD600–570), after the addition of resazurin (0.001% final concentration) and incubation at 35 °C for additional 2 h. The absorbance was measured using a Biotek Synergy HTX plate reader.
The percentage of growth inhibition was calculated for each well, using the negative control (media only) and positive control (fungi without inhibitors) on the same plate. The MIC was determined as the lowest concentration, at which the growth was fully inhibited, defined by an inhibition ≥ 80% for C. albicans and an inhibition ≥ 70% for C. neoformans. Due to a higher variance in growth and inhibition, a lower threshold was applied to the data for C. neoformans. In addition, the maximal percentage of growth inhibition was reported as DMax, indicating any compounds with marginal activity. Hits were classified by MIC ≤ 16 µg/mL or MIC ≤ 10 µM in either replicate (n = 2 on different plates).
3. Results and Discussion
Chemistry
Esters of dihydrobetulinic, ursolic, and oleanolic acids 2, 5, and 8 were prepared via the acetate protection of the 3-OH group in native triterpenoid acids and subsequent transformation of the resulting acetates 1, 4, and 7 to unstable acyl chlorides. Then the acyl chlorides were involved without further purification in the reaction with tris(hydroxymethyl)aminomethane under reported conditions [7]. The reactions afforded the desired esters 2, 5, and 8 in 15%–23% yields and amides 5a and 8a in 24%–32% yields. The resulting compounds were separated by column chromatography (Scheme 1). Guanylation of the amine group was carried out by a standard procedure [16] on treatment with commercially available guanylation reagent 1H-pyrazole-1-carboxamidine hydrochloride.
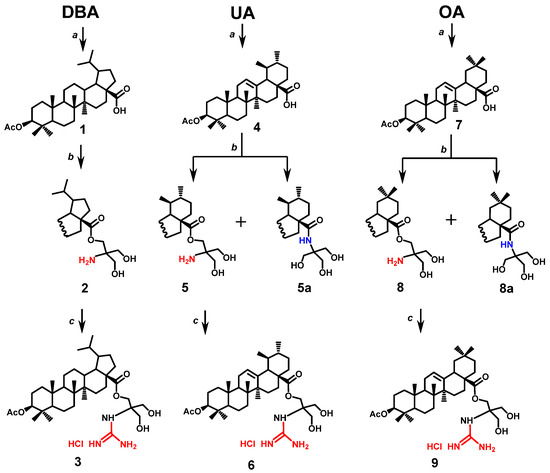
Scheme 1.
Synthesis of compounds 2, 3, 5, 5a, 6, 8, 8a and 9. Reagents and conditions: (a) AcCl, THF, Py, dimethylaminopyridine (DMAP), r.t.; (b) 1. (COCl)2, CH2Cl2; 2. tris(hydroxymethyl)aminomethane, DMAP, Py, CH2Cl2, r.t.; (c) 1H-Pyrazole-1-carboxamidine hydrochloride, N,N-diisopropylethylamine (DIPEA), DMF, r.t., 24 h.
All compounds prepared in this study, including NVX-207, were tested in vitro for their cytotoxic activity against human tumor cell lines: Jurkat (T-lymphoblastic leukemia), K562 (chronic myeloid leukemia), and U937 (histiocytic lymphoma). These compounds were tested in triplicates by standard MTS assay.
The introduction of the tris(hydroxymethyl)aminomethane moiety into the molecules of triterpene acids 1, 4, and 7 markedly enhanced the cytotoxic activity of the resulting conjugates 2, 5, 8 as well as their guanidinium derivatives 3, 6, and 9 against all tested cell lines irrespective of the triterpene skeleton type (Table 1).

Table 1.
Cytotoxicity of dihydrobetulinic-, ursolic-, oleanolic acids and compounds NVX-207, 2, 3, 5, 6, 8, and 9 against Jurkat, K562, and U937 cancer cells.
For example, the IC50 values of compounds 2 and 3 were 2.8 and 3.1 µM for T-lymphoblastic leukemia cells and 3.2 and 2.3 µM for chronic myeloid leukemia cells, while IC50 values of dihydrobetulinic acid were 59 and 44 µM, respectively. The most pronounced differences in the antitumor activity were found for oleanolic acid and its conjugates 8 and 9. Indeed, the IC50 values of oleanolic acid, its conjugates 8 and 9 for Jurkat cells were 271, 2.9, and 7.6 µM, respectively.
The identified lead molecule 2 with the highest antitumor characteristics was evaluated for the possible apoptosis induction in Jurkat cells using Annexin V/7-AAD staining [14]. The highest percentage of late apoptosis (91.8%) was detected upon the addition of compound 2 (4 μM) to cells followed by 48 h incubation (Figure 3E).
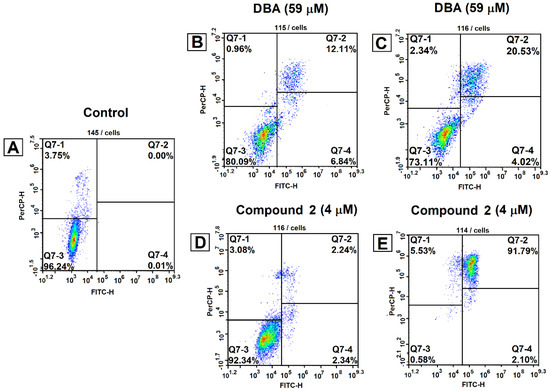
Figure 3.
AnnexinV/7-AAD staining upon induction of apoptosis in Jurkat cells. Cells were treated with compound 2 and dihydrobetulinic acid at their IC50 concentration for 24 and 48 h. Then, the cells were harvested, stained with Annexin V/7-AAD, and analyzed by flow cytometry. The experiments were performed three times, and the results of the representative experiments are shown. The first cytogram represents an untreated cell sample (A); after incubation with dihydrobetulinic acid for 24 h (B) and for 48 h (C); and after incubation with compound 2 for 24 h (D) and for 48 h (E). Q7-1, necrotic cells; Q7-2, late apoptotic cells; Q7-3, living cells; Q7-4, early apoptotic cells.
Dihydrobetulinic acid triggers apoptosis in Jurkat cells at higher doses as compared to ester 2. The number of apoptotic cells on treatment with dihydrobetulinic acid (59 μM) for 48 h constituted around 24% (4.0% of early-stage and 20.5% of secondary necrotic/late-stage apoptotic), while the number of vital cells was 73.1% (Figure 3C).
DNA flow cytometry was also used to analyze the cell cycle kinetics in Jurkat cells pre-incubated with dihydrobetulinic acid and ester 2 at their IC50 concentration for 24 and 48 h (Figure 4).
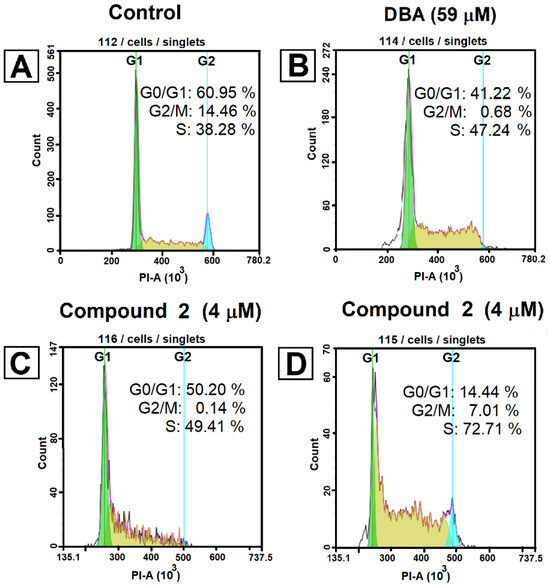
Figure 4.
Cell cycle analysis in Jurkat cells. Jurkat cells were treated with tested compound 2 at the IC50 concentration for 24–48 h and dihydrobetulinic acid for 48 h. The cells were trypsinized, harvested, and washed three times with ice-PBS for PI-stained DNA content detected by flow cytometry. The experiments were performed three times, and the results of the representative experiments are shown. The first cytogram represents an untreated cell sample (A); after incubation with dihydrobetulinic acid for 48 h (B); and after incubation with compound 2 for 24 h (C) and for 48 h (D).
The results of experiments have shown in significant S phase arrest in cells after treatment with dihydrobetulinic acid or compound 2. The ratio of cells in the S phase increased from 38.3% (control) to 72.7% in cells treated with ester 2 (4 μM for 48 h) and increased to 47.2% in cells treated with dihidrobetulinic acid. The appropriate number of cells in the G2/M phase decreased. For example, the treatment of Jurkat cells with ester 2 resulted in a decrease of these cells in the G2/M phase from 14.4% (control) to 7% (Figure 4D). Considering these results, we assume that dihydrobetulinic acid and compound 2 are able to trigger the programmed cell death, including apoptotic mechanisms and cell cycle arrest in the S phase.
Previously, it was shown that triterpene acids, either in a pure state or as parts of plant extracts, exhibit moderate bacteriostatic activity, mainly against Gram-positive bacteria [17,18,19]. The minimum inhibitory concentrations (MICs) of ursolic and oleanolic acids against various Staphylococcus aureus strains, including the methicillin-resistant (MRSA) strain, were in the range from 8 to 64 µg/mL, while betulinic acid was less active against these strains [20]. Some semisynthetic analogues of betulinic, ursolic, and oleanolic acids were synthesized and studied in vitro as potential antimicrobial agents [21,22,23]. We have studied the antibacterial activities of dihydrobetulinic, ursolic, and oleanolic acids and their conjugates 2, 3, 5, 6, 8, and 9 using four bacterial strains, including Gram-negative Escherichia coli, Acinetobacter baumannii, and Pseudomonas aeruginosa and Gram-positive methicillin-resistant Staphylococcus aureus (MRSA). The antifungal activity was determined against Candida albicans and Cryptococcus neoformans. The antimicrobial screening of compounds was performed by the Community for Open Antimicrobial Drug Discovery (CO-ADD, Institute for Molecular Bioscience, University of Queensland, Brisbane, Australia). The primary antimicrobial screening of compounds was performed at a single point concentration of 32 μg/mL. MICs were determined for the “hit” compounds from the primary screening (samples with bacterial growth inhibition value of above 80%).
Our experimental results demonstrated the absence of significant antimicrobial effect of native triterpenoid acids and lupane triterpenoids 2 and 3 (Table 2).

Table 2.
Antimicrobial activities of dihydrobetulinic-, ursolic-, oleanolic acids and compounds 2, 3, 5, 6, 8, and 9 a.
Ursane and oleane conjugates have shown the higher antimicrobial effect than their lupane analogues. Ursolic and oleanolic acid conjugates with guanidinium group 6 and 9 showed a moderate activity against S. aureus (MIC 4 μg/mL). Ursolic ester 5 was highly active against C. albicans and C. neoformans (MICs < 0.25 and 2.0 μg/mL) (Figure 5).
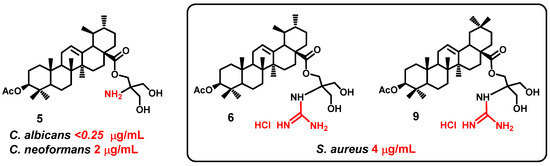
Figure 5.
Structures of lead compounds 5, 6, and 9.
4. Conclusions
In summary, we have synthesized new esters of dihydrobetulinic, ursolic, and oleanolic acids with tris(hydroxymethyl)aminomethane moiety at the C-28 position and tested them in vitro for anticancer and antimicrobial activity. The introduction of the TRIS-moiety into the molecules of native triterpene acids markedly enhanced the cytotoxic activity of the resulting conjugates 2, 5, and 8, as well as their guanidinium derivatives 3, 6, and 9 against tumor cell lines Jurkat, K562, and U937, irrespective of the triterpene skeleton type. The ester of dihydrobetulinic acid with tris(hydroxymethyl)aminomethane (2) as a lead molecule with the highest antitumor characteristics was selected for extensive biological testing, which showed that this compound is capable of triggerring programmed cell death, including apoptotic mechanisms and cell cycle arrest in the S phase. Primary antimicrobial screening showed the absence of significant antimicrobial effect of native triterpenoid acids and lupane triterpenoids 2 and 3. Conjugates with the ursane and oleane skeleton type demonstrated a higher antimicrobial effect than their lupane analogues. Ursolic and oleanolic acid conjugates with guanidinium group 6 and 9 displayed moderate activity against S. aureus, while the ursolic ester 5 was highly active against C. albicans and C. neoformans.
Author Contributions
Validation and writing—review and editing, A.S.; performing the chemistry experiments, R.K. and D.N.; performing the biology experiments, L.D. The manuscript was prepared through the contributions of A.S., L.D. and D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed under financial support from the Russian Science Foundation (Grant 19-73-00155).
Acknowledgments
The antimicrobial screening performed by CO-ADD (The Community for Antimicrobial Drug Discovery) was funded by the Wellcome Trust (UK) and The University of Queensland (Australia).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2017, 34, 90–122. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Sarek, J.; Kvasnica, M.; Vlk, M.; Urban, M.; Dzubak, P.; Hajduch, M. The potential of triterpenoids in the treatment of melanoma. In Research on Melanoma: A Glimpse into Current Directions and Future Trends; Murph, M., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 125–158. [Google Scholar]
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543–593. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Moreira, V.M.; Goncalves, B.M.F.; Lealab, A.S.; Jing, Y. Ursane-type pentacyclic triterpenoids as useful platforms to discover anticancer drugs. Nat. Prod. Rep. 2012, 29, 1463–1479. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluderovic, G.N.; Kalbitz, J.; Dräger, B.; Paschke, R. Small structural changes of pentacycliclupane type triterpenoid derivatives lead to significant differences in their anticancer properties. Eur. J. Med. Chem. 2010, 45, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.; Wacheck, V.; Buckley, J.; Nagy, K.; Thalhammer, J.; Paschke, R.; Triche, T.; Jansen, B.; Selzer, E. Characterization of NVX-207, a novel betulinic acid-derived anti-cancer compound. Eur. J. Clin. Investig. 2009, 39, 384–394. [Google Scholar] [CrossRef]
- Bache, M.; Bernhardt, S.; Passin, S.; Wichmann, H.; Hein, A.; Zschornak, M.; Kappler, M.; Taubert, H.; Paschke, R.; Vordermark, D. Betulinic acid derivatives NVX-207 and B10 for treatment of glioblastoma—An in vitro study of cytotoxicity and radiosensitization. Int. J. Mol. Sci. 2014, 15, 19777–19790. [Google Scholar] [CrossRef]
- Liebscher, G.; Vanchangiri, K.; Mueller, T.; Feige, K.; Cavalleri, J.M.; Paschke, R. In vitro anticancer activity of betulinic acid and derivatives thereof on equine melanoma cell lines from grey horses and in vivo safety assessment of the compound NVX-207 in two horses. Chem. Biol. Interact. 2016, 246, 20–29. [Google Scholar] [CrossRef]
- Saczewski, F.; Balewski, L. Biological activities of guanidine compounds. Expert Opin. Ther. Pat. 2009, 19, 1417–1448. [Google Scholar] [CrossRef]
- Castagnolo, D.; Schenone, S.; Botta, M. Guanylated diamines, triamines, and polyamines: Chemistry and biological properties. Chem. Rev. 2011, 111, 5247–5300. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Pezzuto, J.M.; Pisha, E. Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg. Med. Chem. Lett. 1998, 8, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’yakonov, V.; Dzhemilev, U. Synthesis and Evaluation of Anticancer Activities of Novel C-28 Guanidine-Functionalized Triterpene Acid Derivatives. Molecules 2018, 23, 3000. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping Chemists Discover New Antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef]
- Feichtinger, K.; Zapf, C.; Sings, H.L.; Goodman, M. Diprotected Triflylguanidines: A New Class of Guanidinylation Reagents. J. Org. Chem. 1998, 63, 3804–3805. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evidence-Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and characterisation of antimicrobial properties of semisynthetic betulin derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Mahapatra, A.; Jamil, K.; Reddy, P.S. Antimicrobial Activity of Some Pentacyclic Triterpenes and Their Synthesized 3-O-Lipophilic Chains. Biol. Pharm. Bull. 2004, 27, 1576–1579. [Google Scholar] [CrossRef]
- Innocente, A.; Casanova, B.B.; Klein, F.; Lana, A.D.; Pereira, D.; Muniz, M.N.; Sonnet, P.; Gosmann, G.; Fuentefria, A.M.; Gnoatto, S.C.B. Synthesis of isosteric triterpenoid derivatives and antifungal activity. Chem. Biol. Drug Des. 2014, 83, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, H.; Li, Q.; Wang, D.; Zhang, J.; Hao, X.; Yang, X. Pentacyclic triterpene derivatives possessing polyhydroxyl ring A inhibit Gram-positive bacteria growth by regulating metabolism and virulence genes expression. Eur. J. Med. Chem. 2015, 95, 64–75. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).