Green, Microwave-Assisted Synthesis of O-Perbutyrylated-Alkyl-Glycosides †
Abstract
:1. Introduction
2. Methods
2.1. Chemicals and Equipment
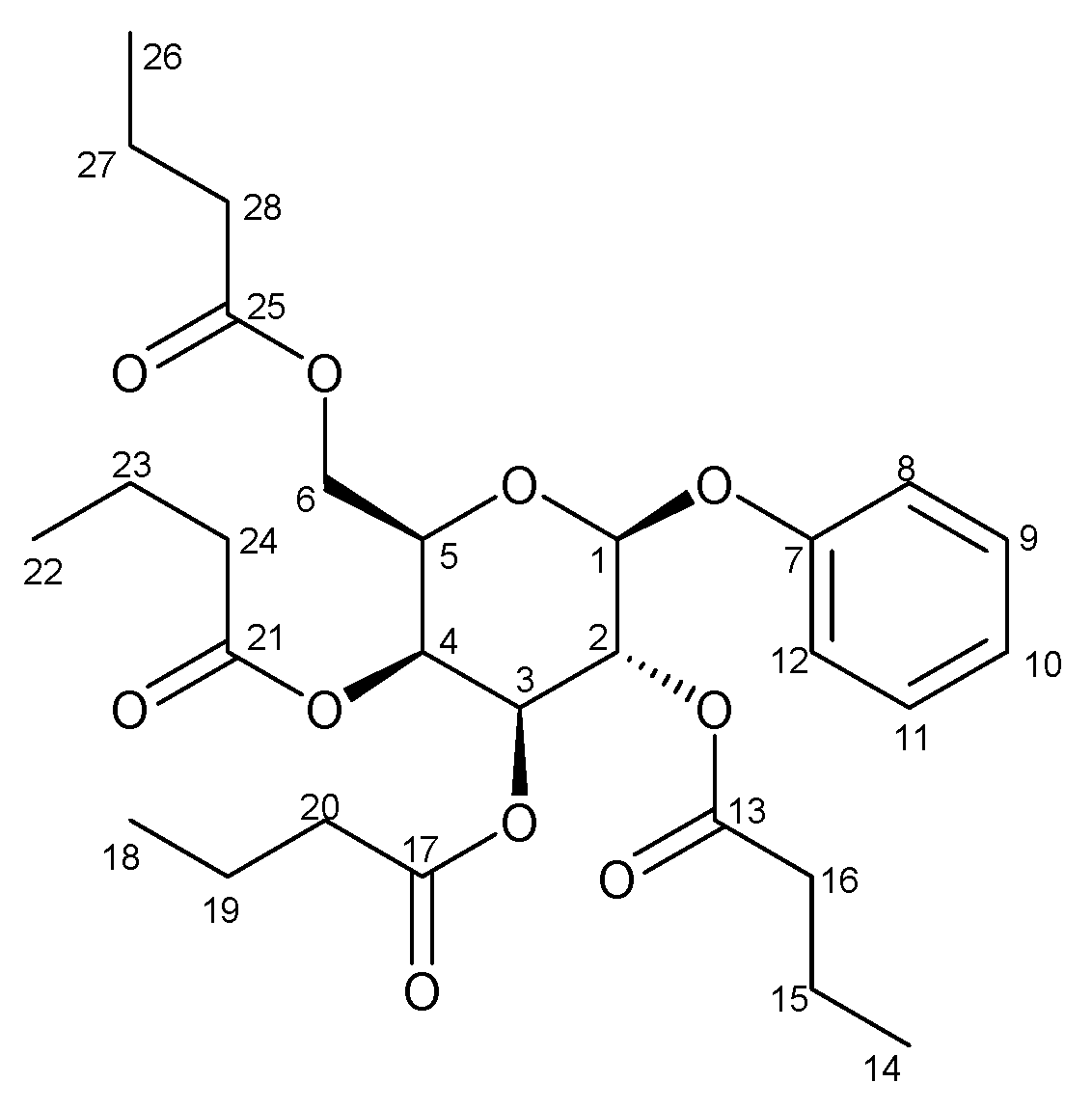
2.2. Synthesis of O-perbutyrylated-phenyl-galactose at Room Temperature
2.3. Synthesis of O-perbutyrylated-phenyl-galactose by Microwave Heating
2.4. Characterization of O-perbutyrylated-phenyl-galactose
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ferrier, R.J. An Historical Overview. In The Organic Chemistry of Sugars, 1st ed.; Levy, D.E., Fügedi, P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 3–24. [Google Scholar]
- Bissaro, B.; Monsan, P.; Faure, R.; O’Donohue, M.J. Glycosynthesis in a waterworld: New insight into the molecular basis of transglycosylation in retaining glycoside hydrolases. Biochem. J. 2015, 467, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Guisan, J.M.; Terreni, M.; Palomo, J.M. Regioselective monodeprotection of peracetylated carbohydrates. Nat. Protoc. 2012, 7, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Wang, J.L. Microbial degradation of pyridine by Paracoccus sp. isolated from contaminated soil. J. Hazard. Mater. 2010, 176, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kumar, R.; Maulik, P.R.; Misra, A.K. Efficient acetylation of carbohydrates promoted by imidazole. Eur. J. Org. Chem. 2005, 2005, 4265–4270. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green solvents in carbohydrate chemistry: From raw materials to fine chemicals. Chem. Rev. 2015, 115, 6811–6843. [Google Scholar] [CrossRef] [PubMed]
- Carroux, C.J.; Moeker, J.; Motte, J.; Lopez, M.; Bornaghi, L.F.; Katneni, K.; Ryan, E.; Morizzi, J.; Shackleford, D.M.; Charman, S.A.; et al. Synthesis of acylated glycoconjugates as templates to investigate in vitro biopharmaceutical properties. Bioorg. Med. Chem. Lett. 2013, 23, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zi, C.T.; Yang, D.; Dong, F.W.; Li, G.T.; Li, Y.; Ding, Z.T.; Zhou, J.; Jiang, Z.H.; Hu, J.M. Synthesis and antitumor activity of novel per-butyrylated glycosides of podophyllotoxin and its derivatives. Bioorg. Med. Chem. 2015, 23, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Escalante, E.; González-Olivares, L.G.; Castañeda-Ovando, A.; Salazar-Pereda, V.; Trant, J.F.; Bautista-Ávila, M.; Alatorre-Santamaría, S. Green, Microwave-Assisted Synthesis of O-Perbutyrylated-Alkyl-Glycosides. Proceedings 2019, 41, 42. https://doi.org/10.3390/ecsoc-23-06500
Pérez-Escalante E, González-Olivares LG, Castañeda-Ovando A, Salazar-Pereda V, Trant JF, Bautista-Ávila M, Alatorre-Santamaría S. Green, Microwave-Assisted Synthesis of O-Perbutyrylated-Alkyl-Glycosides. Proceedings. 2019; 41(1):42. https://doi.org/10.3390/ecsoc-23-06500
Chicago/Turabian StylePérez-Escalante, Emmanuel, Luis Guillermo González-Olivares, Araceli Castañeda-Ovando, Verónica Salazar-Pereda, John F. Trant, Mirandeli Bautista-Ávila, and Sergio Alatorre-Santamaría. 2019. "Green, Microwave-Assisted Synthesis of O-Perbutyrylated-Alkyl-Glycosides" Proceedings 41, no. 1: 42. https://doi.org/10.3390/ecsoc-23-06500
APA StylePérez-Escalante, E., González-Olivares, L. G., Castañeda-Ovando, A., Salazar-Pereda, V., Trant, J. F., Bautista-Ávila, M., & Alatorre-Santamaría, S. (2019). Green, Microwave-Assisted Synthesis of O-Perbutyrylated-Alkyl-Glycosides. Proceedings, 41(1), 42. https://doi.org/10.3390/ecsoc-23-06500





