Microwave Assisted Three Component Reaction Conditions to Obtain New Thiazolidinones with Different Heterocyclic Skeletons †
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Information
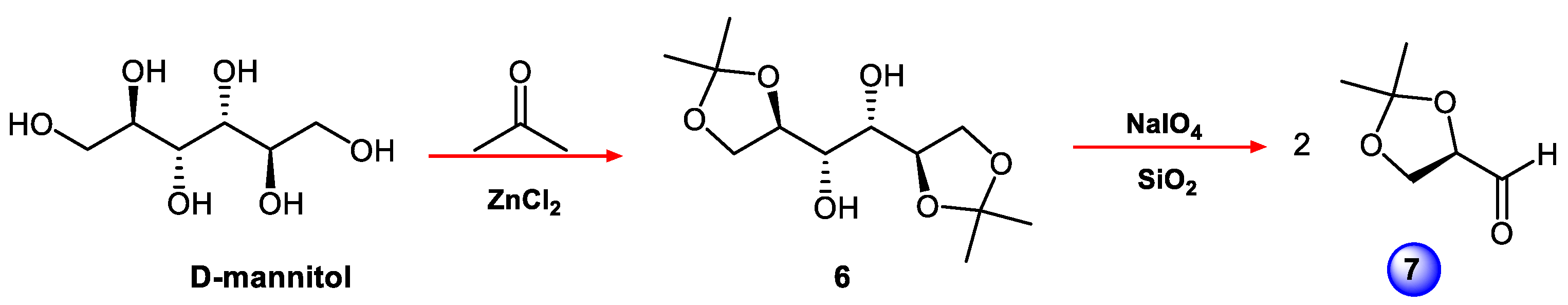
4.2. Synthesis of (R)-2,3-O-isopropiliden-D-glyceraldehyde (7)
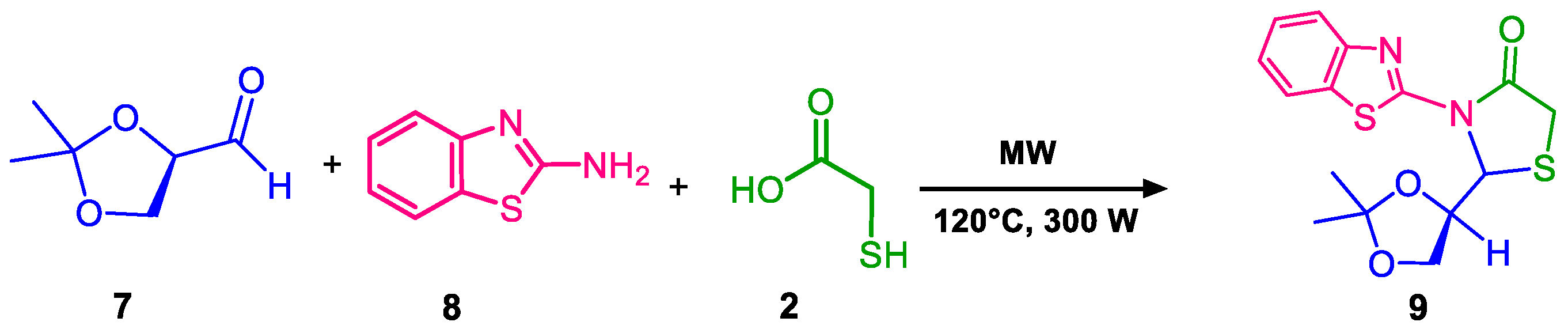
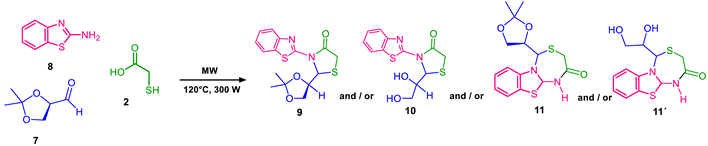
4.3. Microwave Reactions
- 2-(2,2-dimethyl-1,3-dioxolan-4-yl)-3-phenylthiazolidin-4-one (10a): 1H NMR (300 MHz, DMSO-d6) δ 7.55–7.05 (m, 5H), 5.28 (d, J = 7.1 Hz, 1H), 4.75 (c, J = 7.0 Hz, 1H), 3.76 (dd, J = 11.5, 7.1 Hz, 2H), 3.63 (dd, J =11.5, 7.1 Hz 2H), 1.18 (s, 3H), 1.13 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 172.24, 136.41, 129.33, 126.42, 125.39, 109.75, 74.12, 68.61, 66.29, 32.62, 25.73.
- 2-(2,2-dimethyl-1,3-dioxolan-4-yl)-3-(thiazol-2-yl)thiazolidin-4-one (10b): 1H NMR (300 MHz, DMSO-d6) δ 8.09 (d, J = 7.5 Hz, 1H), 7.40 (d, J = 7.5 Hz, 1H), 5.90 (d, J = 7.0 Hz, 1H), 4.85 (c, J = 7.0 Hz, 1H), 3.87 (dd, J = 11.5, 7.1 Hz, 2H), 3.52 (dd, J = 11.5, 7.1 Hz, 2H), 1.38 (s, 3H), 1.33 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 173.05, 154.76, 134.47, 112.73, 109.64, 74.24, 68.87, 68.46, 33.40, 26.09, 25, 28.
- 2-(2,2-dimethyl-1,3-dioxolan-4-yl)-3-(4-methyl-1H-pyrazol-3-yl)thiazolidin-4-one (10c): 1H NMR (300 MHz, DMSO-d6) δ 12.53 (s, 1H), 7.18 (s, 1H), 5.10 (d, J = 7.1 Hz, 1H), 4.71 (c, J = 7.0 Hz, 1H), 3.96 (dd, J = 11.5, 7.1 Hz, 2H), 3.73 (dd, J =11.5, 7.1 Hz, 2H), 3.42 (s, 3H), 1.20 (s, 3H), 1.15 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 175.87, 141.61, 136.33, 109.64, 107.01, 74.24, 68.46, 66.25, 33.40, 25.80, 25.12, 12.84.
- 3-(benzo[d]thiazol-2-yl)-2-(2,2-dimethyl-1,3-dioxolan-4-yl)thiazolidin-4-one (10d): 1H NMR (300 MHz, DMSO-d6) δ 7.65–6.98 (m, 4H), 5.35 (d, J = 7.0 Hz, 1H), 4.49 (c, J = 7.1 Hz, 1H), 4.01 (dd, J = 11.4, 7.0 Hz, 2H), 3.75 (dd, J = 11.5, 7.2 Hz, 2H), 1.21 (s, 3H), 1.19 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 170.92, 166.87, 153.26, 131.37, 125.87, 122.27, 121.26, 118.22, 81,80, 79.57, 67,83, 38.88, 38.61, 38.32.
Funding
Conflicts of Interest
References
- Glycobiology; Oxford University Press: Oxford, UK, 2019.
- Ajay Kumar, K.; Renuka, N.; Raghavendra, K.; Vasanth Kumar, G.; Ranjitha, B.K. New insights to the chemistry of benzothiazepines-an overview. JCS 2015, 5, 79–88. [Google Scholar]
- Metwally, N.H.; El-Doseky, I.A. Facile Synthesis of Some New Pyrazole-Based 2-Thioxo-4-thiazolidinones. Synth. Commun. 2015, 45, 2683–2690. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Leja, M.L.; Kaminskyy, D.V.; Binduga, U.E.; Pinyazhko, R.; Lesyk, R.B.; Gmiński, J. Study of novel anticancer 4-thiazolidinone derivatives. Chem. Biol. Interact. 2017, 262, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Djukic, M.; Fesatidou, M.; Xenikakis, I.; Geronikaki, A.; Angelova, V.T.; Savic, V.; Pavlica, M. In vitroantioxidant activity of thiazolidinone derivatives of 1,3-thiazole and1,3,4-thiadiazole. Chem. Biol. Interact. 2018, 286, 119–131. [Google Scholar] [CrossRef]
- Tageldin, G.N.; Fahmy, S.M.; Ashour, H.M.; Khalil, M.A.; Nassra, R.A.; Labouta, I.M. Design, synthesis and evaluation of some pyrazolo[3,4-d]pyrimidinederivatives bearing thiazolidinone moiety as anti-inflammatory agents. Bioorg. Chem. 2018, 80, 164. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, R.; Jadhav, S.; Makwana, N.; Desai, D.; Chaturbhuj, D.; Sonawani, A.; Paranjape, R. Evaluation of 4-thiazolidinone derivatives as potential reverse transcriptase inhibitors against HIV-1 drug resistant strains. Bioorg. Chem. 2017, 71, 211–218. [Google Scholar] [CrossRef]
- Zhu Qian, J.; Wang, W.M. Multicomponent Reactions in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Design and Synthesis of New Classes of Heterocyclic C-Glycoconjugates and Carbon-Linked Sugar and Heterocyclic Amino Acids by Asymmetric Multicomponent Reactions (AMCRs). Acc. Chem. Res. 2006, 39, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, A.A.; Costilla, I.O.; Lorenzo, F.J.; Calmels, J.J.; Villafain, D.; Mandolesi, S.D.; D’Accorso, N.B.; Ocampo, R.A. Study of the effect of solvent and different Lewis acids in one pot multicomponent microwave assisted reactions for the chemoselective generation of 1.4-thiazepan-3-ones and 4-tiazolidinones. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry Session Polymer and Supramolecular Chemistry, 1–30 November 2017. [Google Scholar] [CrossRef][Green Version]
- Albrakaty, R.H.; Wazzan, N.A.; Obot, I.B. Investigation on Corrosion Inhibition of Mild Steel by Sinapine Thiocyanate in H2SO4 Solution. Int. J. Electrochem. Sci. 2018, 13, 3535. [Google Scholar]
- Danaee, I.; Gholami, M.; RashvandAvei, M.; Maddahy, M.H.J. Quantum chemical and experimental investigations on inhibitory behavior of amino–imino tautomeric equilibrium of 2-aminobenzothiazole on steel corrosion in H2SO4 solution. Ind. Eng. Chem. 2015, 26, 81. [Google Scholar] [CrossRef]
- Alvarenga, E.S.; Texeira Carneiro, V.M.; Oliveira Silverio, F.; Argolo Salibaa, W. High yield synthesis of 1,2:5,6-DI-O-isopropylidene-d-mannitol. J. Chil. Chem. Soc. 2006, 51, 986–988. [Google Scholar] [CrossRef]
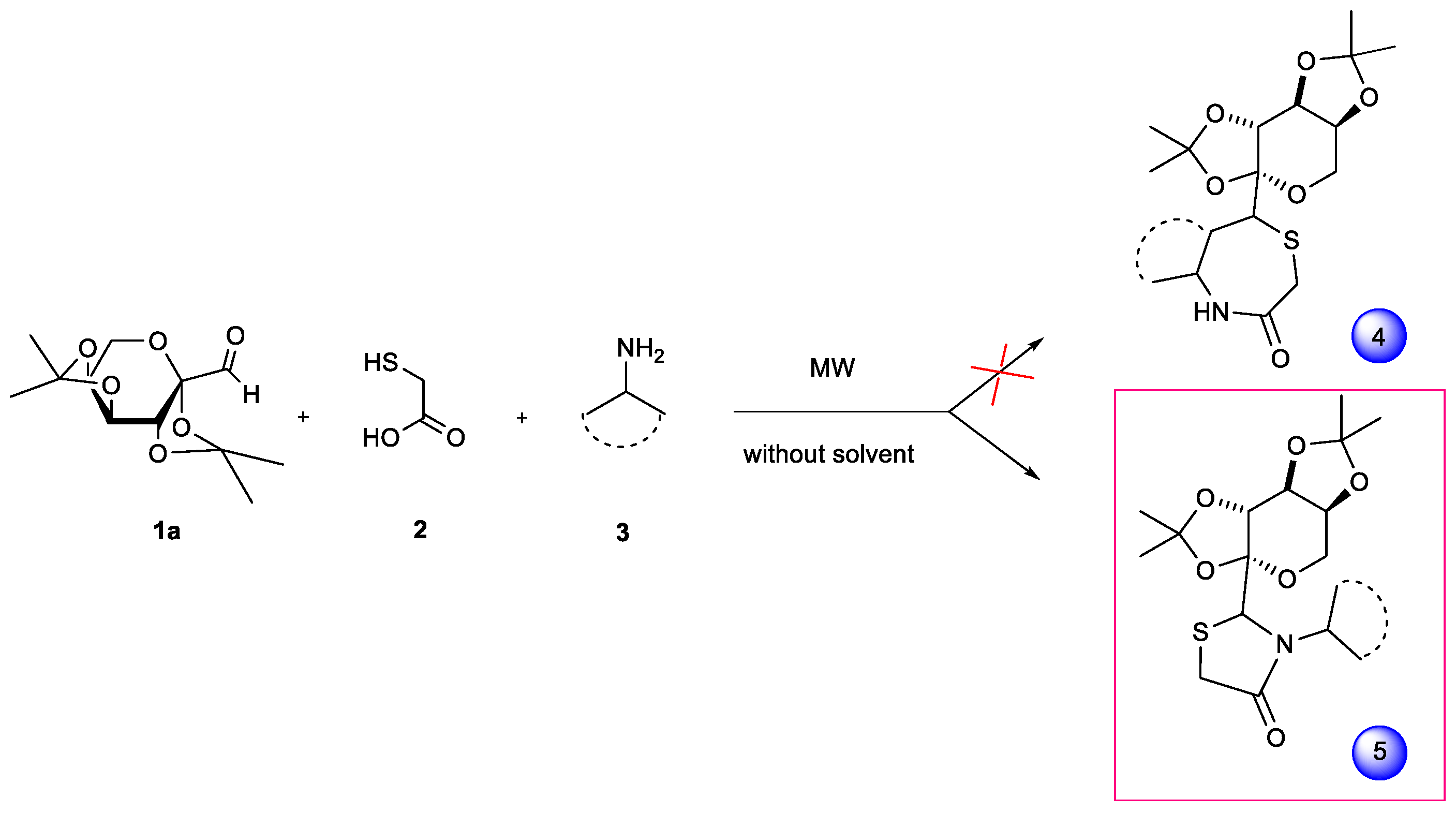

 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | EtOH (eq) | 7:8 (eq) | Mercapto Acetic Acid (eq) | Intervals of Irradiation (min) | Total Irradiation Time (min) | Product/s(% yield) a | d.r. b |
| 1 | 1 | 1:1 | 10 | 10 | 30 | 11 (95%) | --- |
| 2 | 1 | 1:1 | 10 | 30 | 60 | 11 (80%) + 11’ (7%) | --- |
| 3 | 1 | 1:1 | 1 | 10 | 30 | 9 (75%) | 32:68 |
| 4 | 1 | 1:1 | 1 | 30 | 60 | 10 (79%) | 37:63 |
| 5 | --- | 1:1 | 1 | 10 | 30 | 9 (69%) | 41:59 |
| Entry | Product | Yield (%) a | d.r. b |
|---|---|---|---|
| 1 | 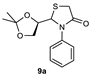 | 82 | 85:15 |
| 2 | 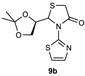 | 69 | 78:22 |
| 3 | 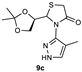 | 73 | 63:37 |
| 4 |  | 75 | 68:32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, F.J.; Ocampo, R.A.; Mandolesi, S.D. Microwave Assisted Three Component Reaction Conditions to Obtain New Thiazolidinones with Different Heterocyclic Skeletons. Proceedings 2019, 41, 38. https://doi.org/10.3390/ecsoc-23-06474
Lorenzo FJ, Ocampo RA, Mandolesi SD. Microwave Assisted Three Component Reaction Conditions to Obtain New Thiazolidinones with Different Heterocyclic Skeletons. Proceedings. 2019; 41(1):38. https://doi.org/10.3390/ecsoc-23-06474
Chicago/Turabian StyleLorenzo, Fernando J., Romina A. Ocampo, and Sandra D. Mandolesi. 2019. "Microwave Assisted Three Component Reaction Conditions to Obtain New Thiazolidinones with Different Heterocyclic Skeletons" Proceedings 41, no. 1: 38. https://doi.org/10.3390/ecsoc-23-06474
APA StyleLorenzo, F. J., Ocampo, R. A., & Mandolesi, S. D. (2019). Microwave Assisted Three Component Reaction Conditions to Obtain New Thiazolidinones with Different Heterocyclic Skeletons. Proceedings, 41(1), 38. https://doi.org/10.3390/ecsoc-23-06474





