Antioxidant Activity of Biphenolic Compounds Anchored on Mesoporous Alumina †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Biphenolic Compounds
2.2. Characterization of Biphenolic Compounds
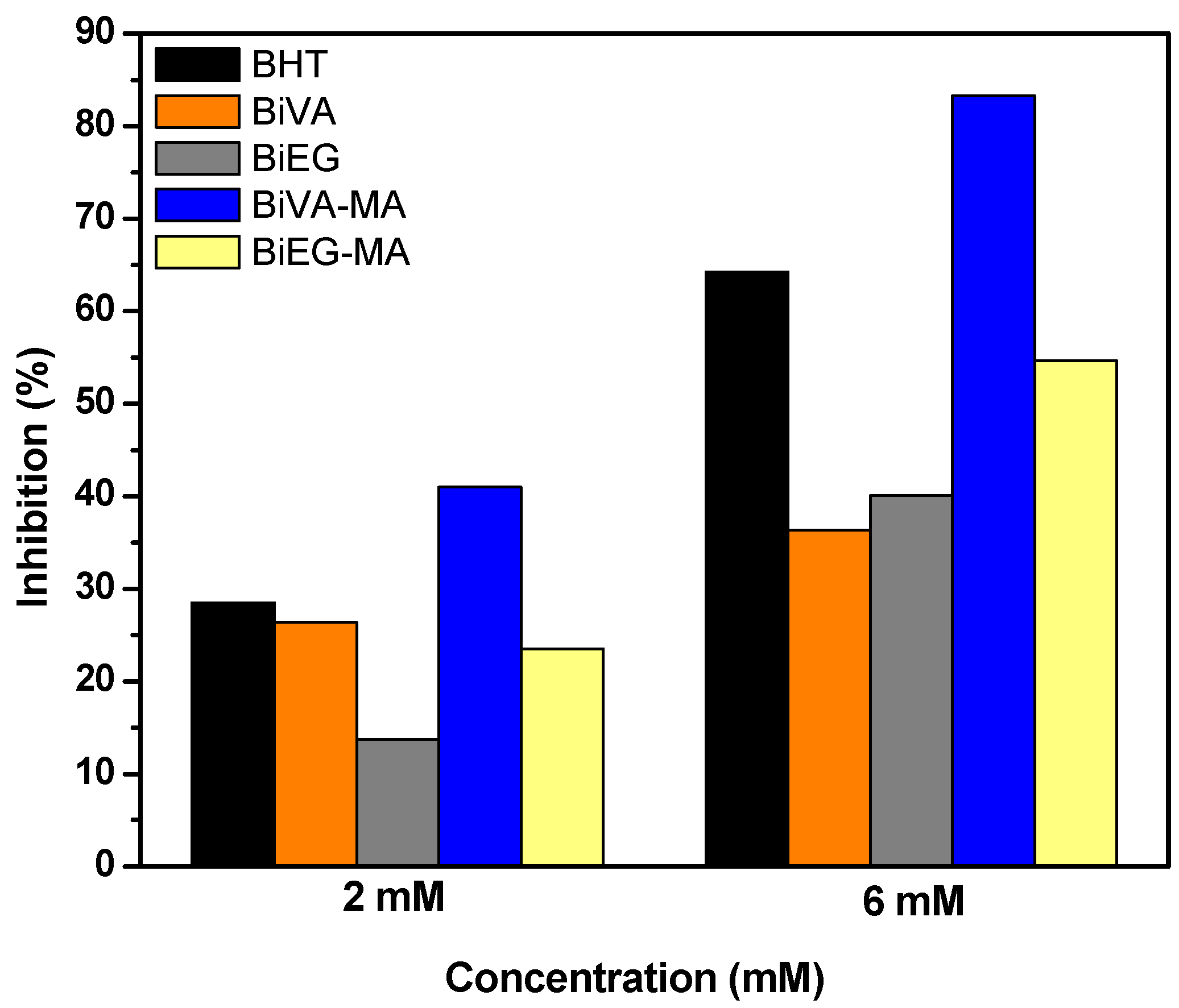
2.3. Determination of Antioxidant Activity
2.4. Characterization of Materials
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Kadoma, Y. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology 2002, 177, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, C.; Yu, J.; Gao, S.; Xu, G. Synthesis of texture-excellent mesoporous alumina using PEG1000 as structure-directing agent. Chin. J. Chem. Eng. 2016, 25, 137–141. [Google Scholar] [CrossRef]
- Tao, Z. Mesoporous silica-based nanodevices for biological applications. RSC Adv. 2014, 4, 18961–18980. [Google Scholar] [CrossRef]
- Farias Dias, “An Improved High Yield Synthesis Of Dehydrodieugenol. Phytochem. Lett. 1988, 27, 3008–3009. [CrossRef]
- Costero, A.M.; Gil, S.; Parra, M.; Mancini, P.M.E.; Kneeteman, M.N.; Quindt, M.I. 5, 5 0-Bis-vanillin derivatives as discriminating sensors for trivalent cations. Tetrahedron Lett. 2015, 56, 3988–3991. [Google Scholar] [CrossRef]
- Cai, W.; Yu, J.; Anand, C.; Vinu, A.; Jaroniec, M. Facile Synthesis of Ordered Mesoporous Alumina and Alumina-Supported Metal Oxides with Tailored Adsorption and Framework Properties. Chem. Mater. 2011, 23, 1147–1157. [Google Scholar] [CrossRef]
- Guntero, V.A.; Ferretti, C.A.; Mancini, P.M.E.; Kneeteman, M.N. Encapsulation Of Compounds Biphenyls Into Sba-15: Synthesis of Composites for Application. Glob. J. Eng. Sci. Res. 2016, 5, 32–37. [Google Scholar]
- Alam, N.M.; Bristi, N.J.; Rafiquzzama, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
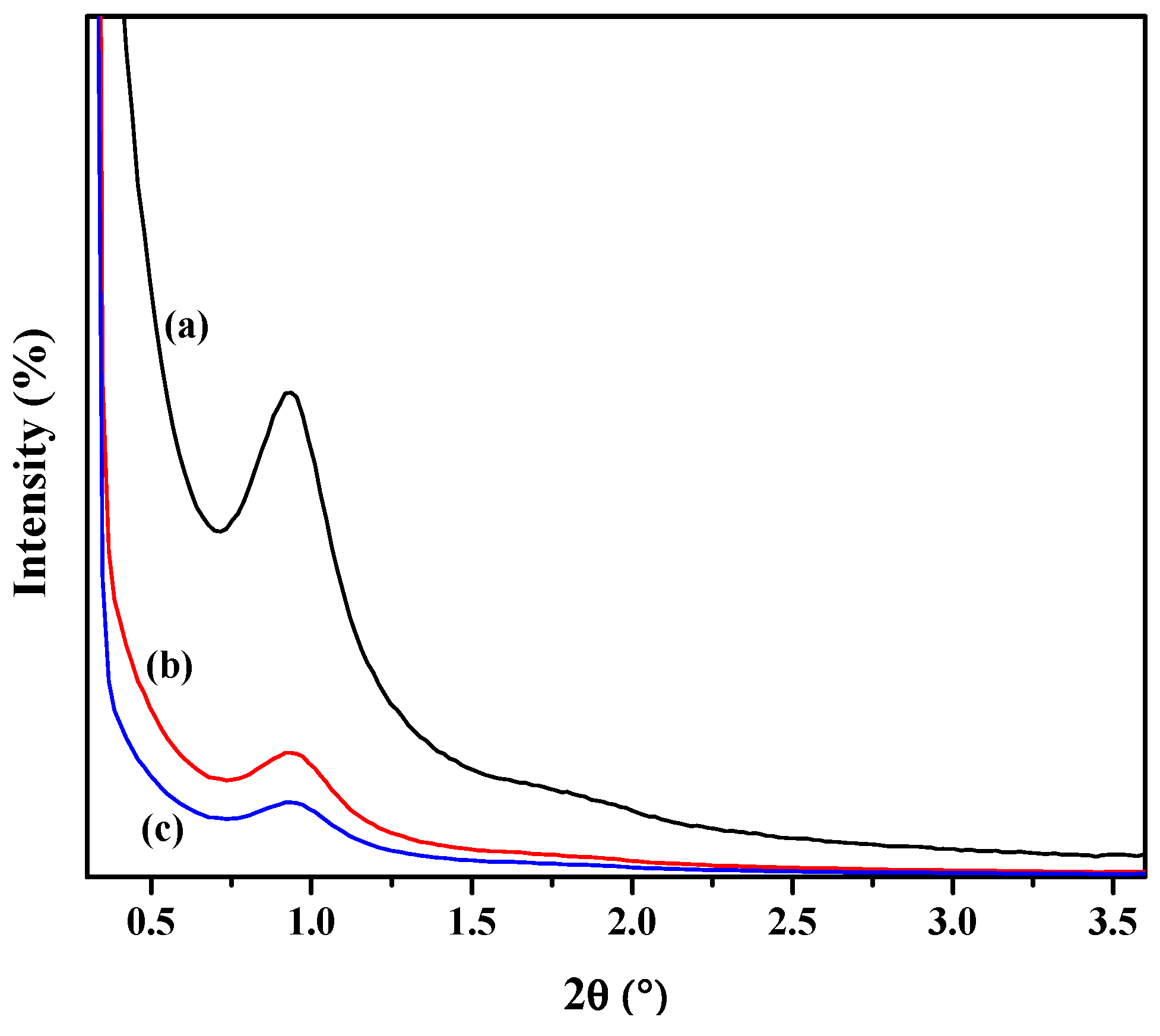
| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (Å) |
|---|---|---|---|
| MA | 266 | 0.90 | 136 |
| BiVA-MA | 180 | 0.10 | 22 |
| BiEG-MA | 157 | 0.54 | 158 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guntero, V.A.; Ferretti, C.A.; Ormachea, C.; Mancini, P.M.E.; Kneeteman, M.N. Antioxidant Activity of Biphenolic Compounds Anchored on Mesoporous Alumina. Proceedings 2019, 41, 37. https://doi.org/10.3390/ecsoc-23-06498
Guntero VA, Ferretti CA, Ormachea C, Mancini PME, Kneeteman MN. Antioxidant Activity of Biphenolic Compounds Anchored on Mesoporous Alumina. Proceedings. 2019; 41(1):37. https://doi.org/10.3390/ecsoc-23-06498
Chicago/Turabian StyleGuntero, Vanina A., Cristián A. Ferretti, Carla Ormachea, Pedro M. E. Mancini, and María N. Kneeteman. 2019. "Antioxidant Activity of Biphenolic Compounds Anchored on Mesoporous Alumina" Proceedings 41, no. 1: 37. https://doi.org/10.3390/ecsoc-23-06498
APA StyleGuntero, V. A., Ferretti, C. A., Ormachea, C., Mancini, P. M. E., & Kneeteman, M. N. (2019). Antioxidant Activity of Biphenolic Compounds Anchored on Mesoporous Alumina. Proceedings, 41(1), 37. https://doi.org/10.3390/ecsoc-23-06498





