In-Situ Preparation of Three Types of Noble Metal Nanoparticles-Polyacrylonitrile Nanofibers (NM NPs-PAN NFs, M = Pt, Au, and Ag) Using Electrospinning Technique Assisted with Ultrasound Irradiation †
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Procedure
3. Result and Discussion
3.1. XRD Patterns
3.2. Morphology Studies
3.3. FT-IR Studies
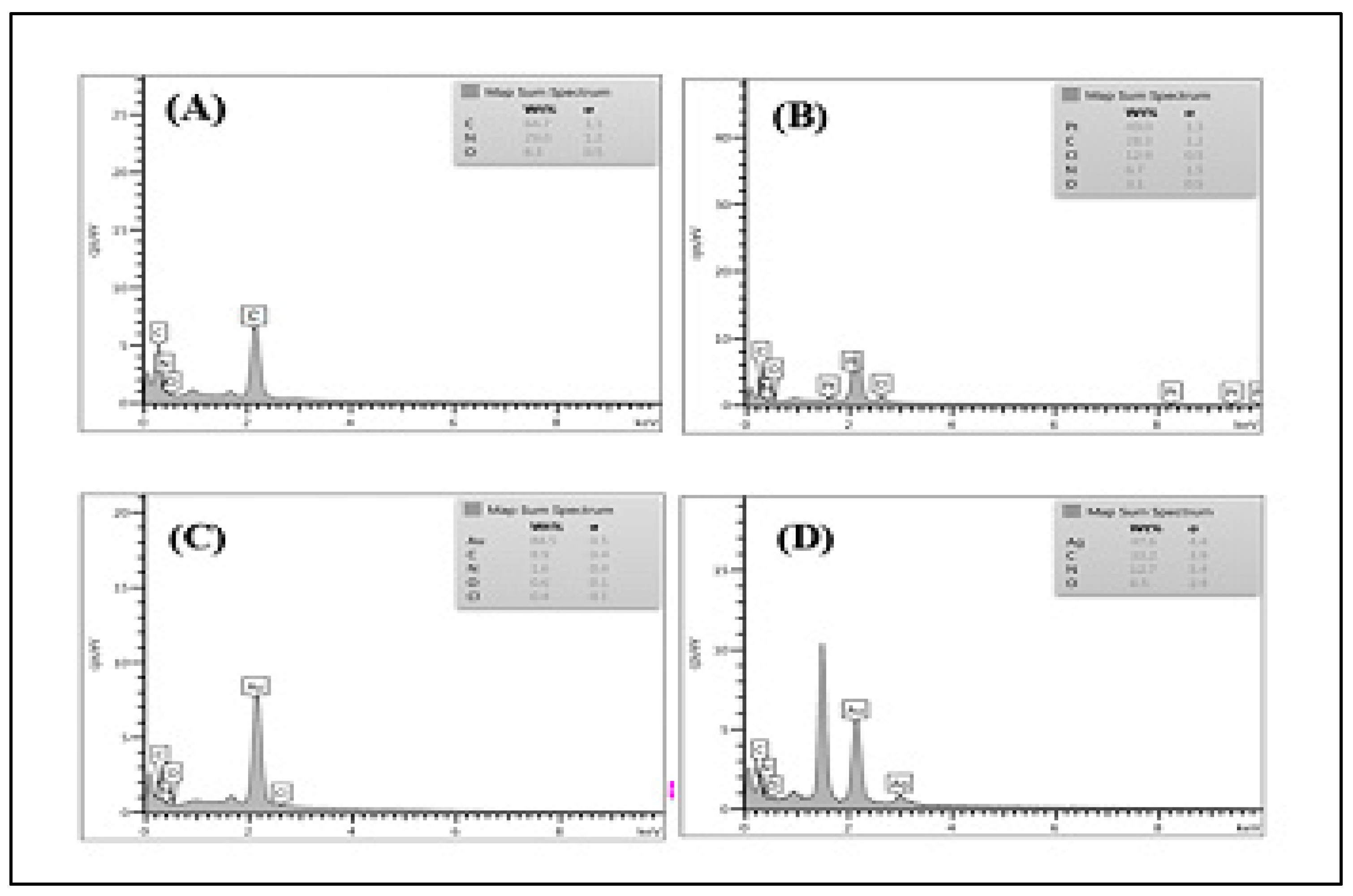
3.4. Elemental Analysis Study
4. Conclusions
References
- Bounor-Legare, V.; Cassagnau, P. In situ synthesis of organic–inorganic hybrids or nanocomposites from sol–gel chemistry in molten polymers. Prog. Polym. Sci. 2014, 39, 1473–1497. [Google Scholar] [CrossRef]
- He, J.; Kunitake, T.; Nakao, A. Facile in Situ Synthesis of Noble Metal Nanoparticles in Porous Cellulose Fibers. Chem. Mater. 2003, 15, 4401–4406. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Zhang, Y.; Su, X.; Huang, Y.; Zhang, T. In situ synthesis of metal clusters encapsulated within small-pore zeolites via a dry gel conversion method. Nanoscale 2018, 10, 11320–11327. [Google Scholar] [PubMed]
- Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic⁻Polymer Nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khanna, P. In situ synthesis of silver nano-particles in polymethylmethacrylate. Mater. Chem. Phys. 2007, 104, 367–372. [Google Scholar]
- Guo, Q.; Ghadiri, R.; Weigel, T.; Aumann, A.; Gurevich, E.L.; Esen, C.; Medenbach, O.; Cheng, W.; Chichkov, B.; Ostendorf, A. Comparison of in Situ and ex Situ Methods for Synthesis of Two-Photon Polymerization Polymer Nanocomposites. Polymers 2014, 6, 2037–2050. [Google Scholar]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I. Nanostructured biocomposites from aliphatic polyesters and bacterial cellulose. Ind. Crop. Prod. 2016, 93, 251–266. [Google Scholar] [CrossRef]
- Zeng, F.-R.; Xu, J.; Xiong, Q.; Qin, K.-X.; Xu, W.-J.; Wang, Y.-X.; Liu, Z.-J.; Li, Z.-L.; Li, Z.-C. Aliphatic polyketones via cross-metathesis polymerization: Synthesis and post-polymerization modification. Polymers 2019, 185, 121936. [Google Scholar] [CrossRef]
- Carrado, K.A.; Xu, L. In Situ Synthesis of Polymer—Clay Nanocomposites from Silicate Gels. Chem. Mater. 1998, 10, 1440–1445. [Google Scholar] [CrossRef]
- Haider, S.; Kamal, T.; Khan, S.B.; Omer, M.; Haider, A.; Khan, F.U.; Asiri, A.M. Natural polymers supported copper nanoparticles for pollutants degradation. Appl. Surf. Sci. 2016, 387, 1154–1161. [Google Scholar] [CrossRef]
- Tian, J.; Jin, J.; Zheng, F.; Zhao, H. Self-Assembly of Gold Nanoparticles and Polystyrene: A Highly Versatile Approach to the Preparation of Colloidal Particles with Polystyrene Cores and Gold Nanoparticle Coronae. Langmuir 2010, 26, 8762–8768. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.J.; Dinsmore, A.; Scurrell, M.S. Fabrication of a Metal Nanoparticles and Polymer Nanofibers Composite Material by an in Situ Chemical Synthetic Route. Langmuir 2005, 21, 7964–7967. [Google Scholar] [CrossRef] [PubMed]
- Porel, S.; Singh, S.; Harsha, S.S.; Rao, D.N.; Radhakrishnan, T.P. Nanoparticle-Embedded Polymer: In Situ Synthesis, Free-Standing Films with Highly Monodisperse Silver Nanoparticles and Optical Limiting. Chem. Mater. 2005, 17, 9–12. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. In situ synthesis of copper nanoparticles and poly(o-toluidine): A metal–polymer composite material. Eur. Polym. J. 2006, 42, 670–675. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, C.; Jin, J.; Xu, W.; Wu, Q.; Gu, J.; Xu, X. Development of a Novel Mixed Titanium, Silver Oxide Polyacrylonitrile Nanofiber as a Superior Adsorbent and its Application for MB Removal in Wastewater Treatment. J. Braz. Chem. Soc. 2018, 29, 560–571. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Alharbi, A.; Khan, W.S.; Swindle, A.; Asmatulu, R. Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials 2015, 8, 7017–7031. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Zhang, Y.; Cheng, N.; Yu, T.; Yang, Y.; Yang, G. Study of carbonization behavior of polyacrylonitrile/tin salt as anode material for lithium-ion batteries. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]



| No. Sample | Position [2 Theta (Degree)] | (h k l) | Crystallite Size (D, nm) |
|---|---|---|---|
| 1 PAN NFs | 17.84, 27.06 | (100), (020) | 3.42 |
| 2 Pt loaded on PAN NFs | 39.92, 46.39, 67.70, 81.07, 86 | (111), (002), (022), (113), (222) | 50.14 |
| 3 Au loaded on PAN NFs | 38.34, 44.44, 64.68, 77.59, 81.93 | (111), (002), (022), (113), (222) | 19.52 |
| 4 Ag loaded on PAN NFs | 38.19, 44.37, 64.55, 77.48, 81.34 | (111), (002), (022), (113), (222) | 24.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, G.K.; Tadjaordi, A. In-Situ Preparation of Three Types of Noble Metal Nanoparticles-Polyacrylonitrile Nanofibers (NM NPs-PAN NFs, M = Pt, Au, and Ag) Using Electrospinning Technique Assisted with Ultrasound Irradiation. Proceedings 2019, 41, 19. https://doi.org/10.3390/ecsoc-23-06488
Kara GK, Tadjaordi A. In-Situ Preparation of Three Types of Noble Metal Nanoparticles-Polyacrylonitrile Nanofibers (NM NPs-PAN NFs, M = Pt, Au, and Ag) Using Electrospinning Technique Assisted with Ultrasound Irradiation. Proceedings. 2019; 41(1):19. https://doi.org/10.3390/ecsoc-23-06488
Chicago/Turabian StyleKara, Gheffar Kh., and Azadeh Tadjaordi. 2019. "In-Situ Preparation of Three Types of Noble Metal Nanoparticles-Polyacrylonitrile Nanofibers (NM NPs-PAN NFs, M = Pt, Au, and Ag) Using Electrospinning Technique Assisted with Ultrasound Irradiation" Proceedings 41, no. 1: 19. https://doi.org/10.3390/ecsoc-23-06488
APA StyleKara, G. K., & Tadjaordi, A. (2019). In-Situ Preparation of Three Types of Noble Metal Nanoparticles-Polyacrylonitrile Nanofibers (NM NPs-PAN NFs, M = Pt, Au, and Ag) Using Electrospinning Technique Assisted with Ultrasound Irradiation. Proceedings, 41(1), 19. https://doi.org/10.3390/ecsoc-23-06488





