A BODIPY-Based Fluorescent Sensor for Amino Acids Bearing Thiol †
Abstract
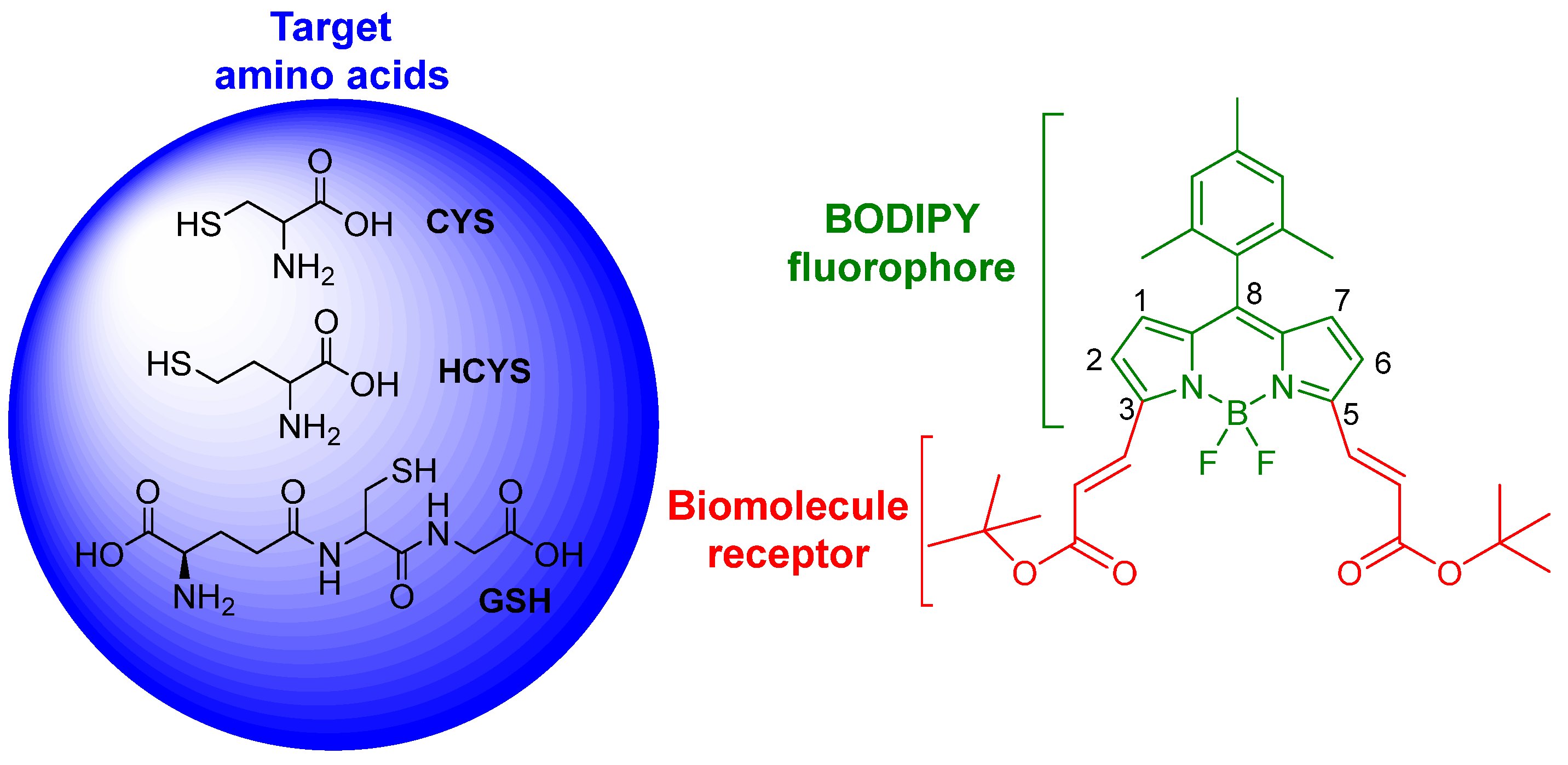
:1. Introduction
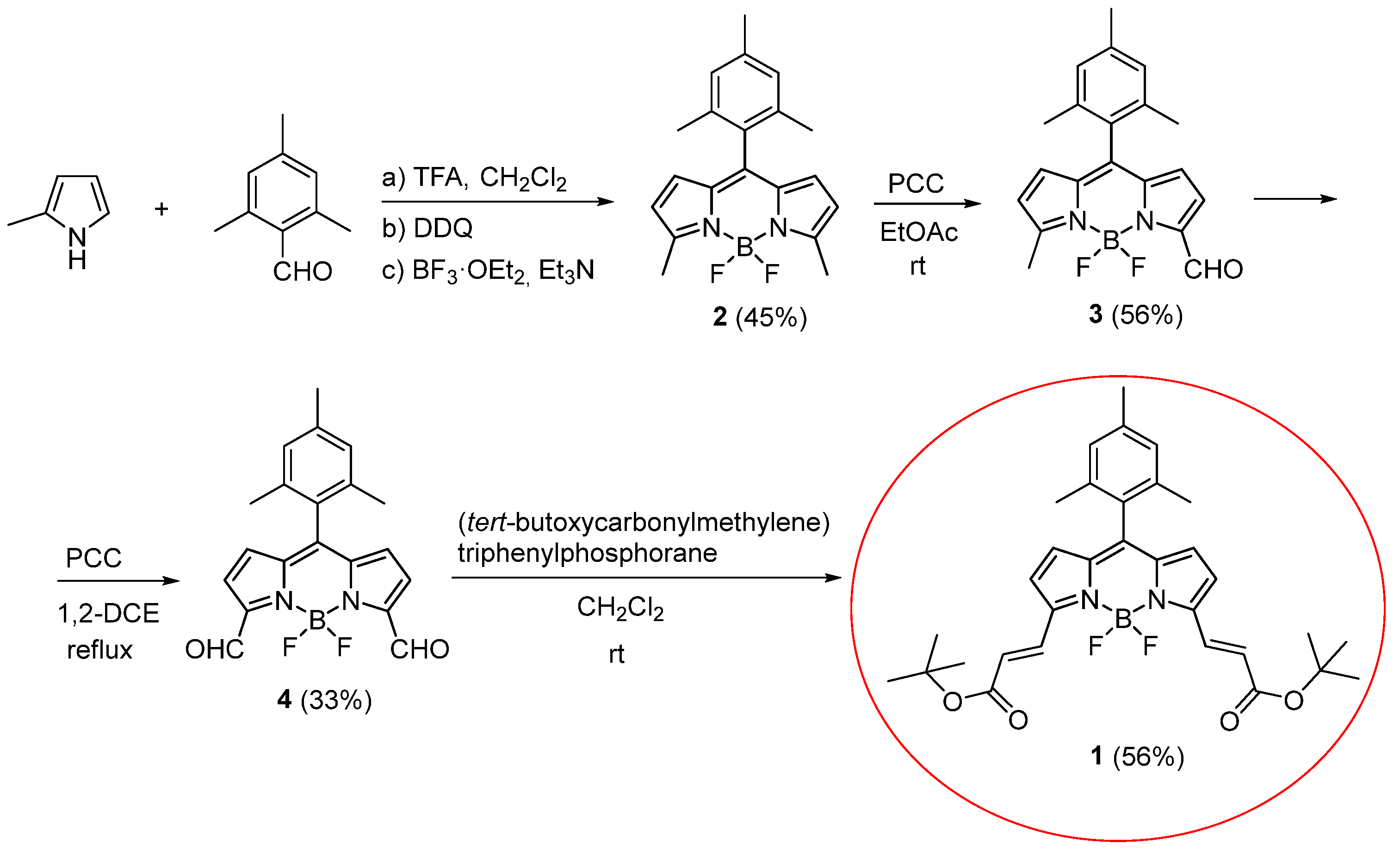
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Ulrich, S.; Dumy, P.; Boturyn, D.; Renaudet, O. Engineering of biomolecules for sensing and imaging applications. J. Drug Deliv. Sci. Technol. 2013, 23, 5–15. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lee, D.; Wang, J.; Li, G.; Yu, J.; Lin, W.; Yoon, J. Development of fluorescent probes based on protection-deprotection of the key functional groups for biological imaging. Chem. Soc. Rev. 2015, 44, 5003–5015. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sibrian-Vazquez, M.; Escobedo, J.O.; Lim, S.; Samoei, G.K.; Strongin, R.M. Homocystamides promote free-radical and oxidative damage to proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 551–554. [Google Scholar] [CrossRef]
- Prasanna de Silva, A.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K.; Resch-Genger, U. Rigidization, preorientation and electronic decoupling—The ‘magic triangle’ for the design of highly efficient fluorescent sensors and switches. Chem. Soc. Rev. 2002, 31, 116–127. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Niu, L.-Y.; Guan, Y.-S.; Chen, Y.-Z.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coord. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Benniston, A.C.; Copley, G. Lighting the way ahead with boron dipyrromethene (Bodipy) dyes. Phys. Chem. Chem. Phys. 2009, 11, 4124–4131. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY dye, the most versatile fluorophore ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent BODIPY dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Clarke, R.G.; Hall, M.J. Recent developments in the synthesis of the BODIPY dyes. Adv. Heterocycl. Chem. 2019, 128, 181–261. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Dehaen, W. Postfunctionalization of the BODIPY core: Synthesis and spectroscopy. Eur. J. Org. Chem. 2015, 6577–6595. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, J.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Kusaka, S.; Sakamoto, R.; Kitagawa, Y.; Okumura, M.; Nishihara, H. An extremely bright heteroleptic bis(dipyrrinato)zinc(II) complex. Chem. Asian J. 2012, 7, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.S. Compensation Film and Organic Dot and Compensation Film. U.S. Patent 15,033,476, 7 May 2015. [Google Scholar]
- Ramos-Torres, A.; Avellanal-Zaballa, E.; Prieto-Castañeda, A.; García-Garrido, F.; Bañuelos, J.; Agarrabeitia, A.R.; Ortiz, M.J. FormylBODIPYs by PCC-promoted selective oxidation of α-methylBODIPYs. Synthethic versatility and applications. Org. Lett. 2019, 21, 4563–4566. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avellanal-Zaballa, E.; Ramos-Torres, Á.; Prieto-Castañeda, A.; García-Garrido, F.; Bañuelos, J.; Agarrabeitia, A.R.; Ortiz, M.J. A BODIPY-Based Fluorescent Sensor for Amino Acids Bearing Thiol. Proceedings 2019, 41, 18. https://doi.org/10.3390/ecsoc-23-06486
Avellanal-Zaballa E, Ramos-Torres Á, Prieto-Castañeda A, García-Garrido F, Bañuelos J, Agarrabeitia AR, Ortiz MJ. A BODIPY-Based Fluorescent Sensor for Amino Acids Bearing Thiol. Proceedings. 2019; 41(1):18. https://doi.org/10.3390/ecsoc-23-06486
Chicago/Turabian StyleAvellanal-Zaballa, Edurne, Ágata Ramos-Torres, Alejandro Prieto-Castañeda, Fernando García-Garrido, Jorge Bañuelos, Antonia R. Agarrabeitia, and María J. Ortiz. 2019. "A BODIPY-Based Fluorescent Sensor for Amino Acids Bearing Thiol" Proceedings 41, no. 1: 18. https://doi.org/10.3390/ecsoc-23-06486
APA StyleAvellanal-Zaballa, E., Ramos-Torres, Á., Prieto-Castañeda, A., García-Garrido, F., Bañuelos, J., Agarrabeitia, A. R., & Ortiz, M. J. (2019). A BODIPY-Based Fluorescent Sensor for Amino Acids Bearing Thiol. Proceedings, 41(1), 18. https://doi.org/10.3390/ecsoc-23-06486





