Abstract
The optimal development of biosensors is a costly and time-consuming task, since an enormous amount of experiments is required. Therefore, the possibility of reusing the biosensors is highly desirable. In this work, a protocol based on the use of formamide for the regeneration of nanophotonic biosensors used for oligonucleotides detection is presented. This protocol was carried out online using the microfluidic system used to drive the target samples to the nanophotonic biosensor, thus allowing the possibility of running several experiments in a row using the same biosensor.
1. Introduction
In the last years, there has been a growing interest in the development of biosensors for different applications in medicine, environmental sensing or food testing, among others [1]. Bridging the gap between the laboratory development of these biosensors to their real application is expensive and time-consuming. Besides their design, manufacture and biofunctionalization, extensive experimental testing work is required. One way to reduce the amount of resources and time required for such testing is by reusing the biosensors. With this aim, different regeneration strategies have been explored by several groups [2,3,4]. However, most regeneration protocols required removing the transducers from the experimental system and reassembling them again in the system after the regeneration was performed.
In this work, we pursued a strategy to reuse silicon-based nanophotonic biosensors functionalized with molecular beacon (MB) probes for the detection of oligonucleotides targets, without removing them from the experimental measuring system. More specifically, in this work we focused on the regeneration of nanophotonic sensors to be used in the detection of microRNA (miRNA) targets. This strategy aims at performing a so-called online regeneration, which not only allows saving time, but also reduces the sensor-to-sensor variance in the experimental sensing results, which is especially useful when testing similar levels of analyte. Chemical regeneration based on formamide (FA) was the strategy explored in this study. FA is a denaturing agent for nucleic acids, which is commonly used in DNA solutions [5]. However, little is known about FA as a denaturing agent for miRNA bound to MB probes immobilized on silicon surfaces, which is what we are interested in [6]. Our study consisted of, after running a typical miRNA sensing experiment, flowing FA into water to dehybridize the probes and regenerate the sensor for performing further experiments.
2. The Biosensor
The principle of operation of the label-free optical biosensor used in this work is based on the interaction of the evanescent wave of a guided mode with the refractive index (RI) changes produced by the sample to be detected in the surroundings of the sensing surface of the nanophotonic structure. In particular, the transducer of this biosensor (sensing structure) was a silicon photonic bandgap (PBG) nanostructure manufactured in a silicon on-insulator (SOI) wafer, as shown in Figure 1a. This nanostructure was created by periodically introducing a modulation in the refractive index of a 1D photonic structure. It consisted of a single mode waveguide placed on top of a silicon oxide lower cladding with straight transversal elements [6,7], as shown in Figure 1b. The photonic detection was based on measuring the shift of the upper edge of the PBG. The bioreceptors employed were MB probes, immobilized by thiol-ene coupling (TEC) chemistry to the previously derivatized surfaces of the PBG sensing structures [8].
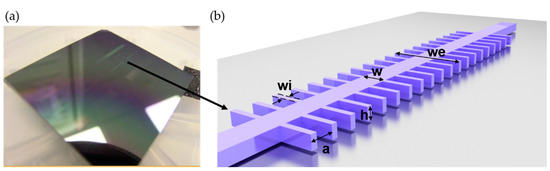
Figure 1.
(a) Photonic chip, manufactured in a silicon on-insulator (SOI) wafer, containing the nanophotonic sensing structures. The black arrow indicates the position where one of the photonic sensing structures is located. (b) Schematic representation of the 1D photonic bandgap (PBG) sensing structure with descriptive key dimensions.
3. Experimental Setup
For testing the biosensors, a polydimethylsiloxane (PDMS) flow cell with a microfluidic channel was attached to the silicon chip to drive the sample to the sensors. The pumping system used was a commercial syringe pump. The optical characterization setup consisted of a continuous sweep tunable laser with the corresponding optical fiber and the optics to transmit and focus the laser light into the access grating couplers. For detection, an objective was used to collect and focus the transmitted light from the output grating couplers into an infrared (IR) camera. The convenient software used to control this setup for optical interrogation was programmed in LabVIEW allowing a continuous acquisition of the spectral response of the sensors.
4. Test and Results
The test consisted of firstly performing an experiment for the photonic detection of the miRNA target (miR-155) that consisted of two steps: first, a saline-sodium citrate (SSC) buffer 5× was flowed to obtain the baseline of the sensing structures; second, a solution of 0.5 μM of miRNA target in SSC 5× was flowed at a flow rate of 20 uL/min over the chip for 15 min. Figure 2a shows the evolution of the PBG edge position during this experiment, where we can see that the PBG was shifted once the miRNA target reached the sensors and that a typical hybridization curve was obtained. Subsequently, the online regeneration test was performed by flowing FA 25% in ultrapure water (UPW) ultrapure water for 20 min (UPW was flowed before and after the FA regeneration for properly washing the surface). The photonic response showed by the sensors during this regeneration protocol was monitored in real-time (see Figure 2b), showing a negative PBG shift during the FA flow that can be ascribed to the dehybridization of the miRNA targets from the MB probes. Finally, the experiment for the detection of miRNA targets was repeated, obtaining the results shown in Figure 2c. Hybridization of the target miRNAs could be observed again, but a signal loss of 50% was obtained after regenerating the sensor.
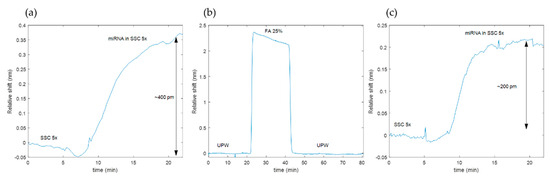
Figure 2.
Time evolution of the PBG edge position of the sensing structure: (a) Acquired during the initial experiment for detection of miRNA; (b) acquired during the online regeneration with formamide (FA) in ultrapure water (UPW); (c) acquired during the second experiment for detection of miRNA (after regeneration).
5. Discussion
These results confirm the feasibility of the online regeneration based on FA, allowing the completion of several experiments in a row. Nevertheless, further optimization work is required to reach a better performance of the protocol. Taking these results as a starting point, different concentrations of FA and different durations of the regeneration period should be explored, as well as the combination of this chemical regeneration with thermal changes that favor the denaturalization of the hybridized oligonucleotides [5].
6. Conclusions
We present a study of a convenient regeneration protocol for nanophotonic biosensors for oligonucleotides detection, which can be easily integrated as an additional step in the experimental microfluidic system required for the proper testing of this type of biosensor. This development saves the time of highly skilled personnel and reduces costs of materials and reagents for the preparation of these biosensors, which is an important advancement in the time-consuming and expensive process of developing and optimizing these biosensors.
Funding
This research was funded by the European Commission through the Horizon 2020 Program (ICT-644242 SAPHELY).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Conroy, P.J.; Hearty, S.; Leonard, P.; O’Kennedy, R.J. Antibody production, design and use for biosensors-based applications. Semin. Cell Dev. Biol. 2009, 20, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Leahy, S.; Lai, Y. Regeneration silicon biosensors through thermal ablation with a hot plate. Sens. Bio-Sens. Res. 2015, 6, 24–27. [Google Scholar] [CrossRef]
- Goode, J.A.; Rushworth, J.V.; Millner, P.A. Sensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2015, 31, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bhatt, V.D.; Märtl, A.; Becherer, M.; Lugli, P. Regenerative, Highly-Sensitive, Non-Ezimatic Dopamine Sensor and Impact of Different Buffer System in Dopamine Sensing. Biosensors 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Dell’Atti, D.; Buhot, A.; Calemczuk, R.; Macini, M.; Livache, T. Effects of formamide on the thermal stability of DNA duplexes on biochips. Anal. Biochem. 2010, 397, 132–134. [Google Scholar] [CrossRef]
- Ruiz-Tórtola, A.; Prats-Quílez, F.; González-Lucas, D.; Bañuls, M.J.; Maquieira, A.; Dalmay, T.; Griol, A.; Hurtado, J.; Bohlmann, H.; Götzen, R.; et al. Experimental study of the evanescent-wave photonic sensors response in presence of molecular beacon conformational changes. J. Biophotonics 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tórtola, A.; Prats-Quílez, F.; González-Lucas, D.; Bañuls, M.J.; Maquieira, A.; Wheeler, G.; Dalmay, T.; Griol, A.; Hurtado, J.; García-Rupérez, J. High sensitivity and label-free oligonucleotides detection using photonic bandgap sensing structures biofunctionalized with molecular beacon probes. Biomed. Opt. Express 2018, 9, 1717–1727. [Google Scholar] [CrossRef]
- González-Lucas, D.; Bañuls, M.J.; García-Rupérez, J.; Maquieira, A. Covalent attachment of biotinylated molecular beacons via thiol-ene coupling. A study on conformational changes upon hybridization and streptavidin binding. Mikrochim. Acta 2017, 184, 3231–3238. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).