A Preliminary Investigation on Human Tears by Means of Surface Enhanced Raman Spectroscopy †
Abstract
:1. Introduction
2. Materials and Methods
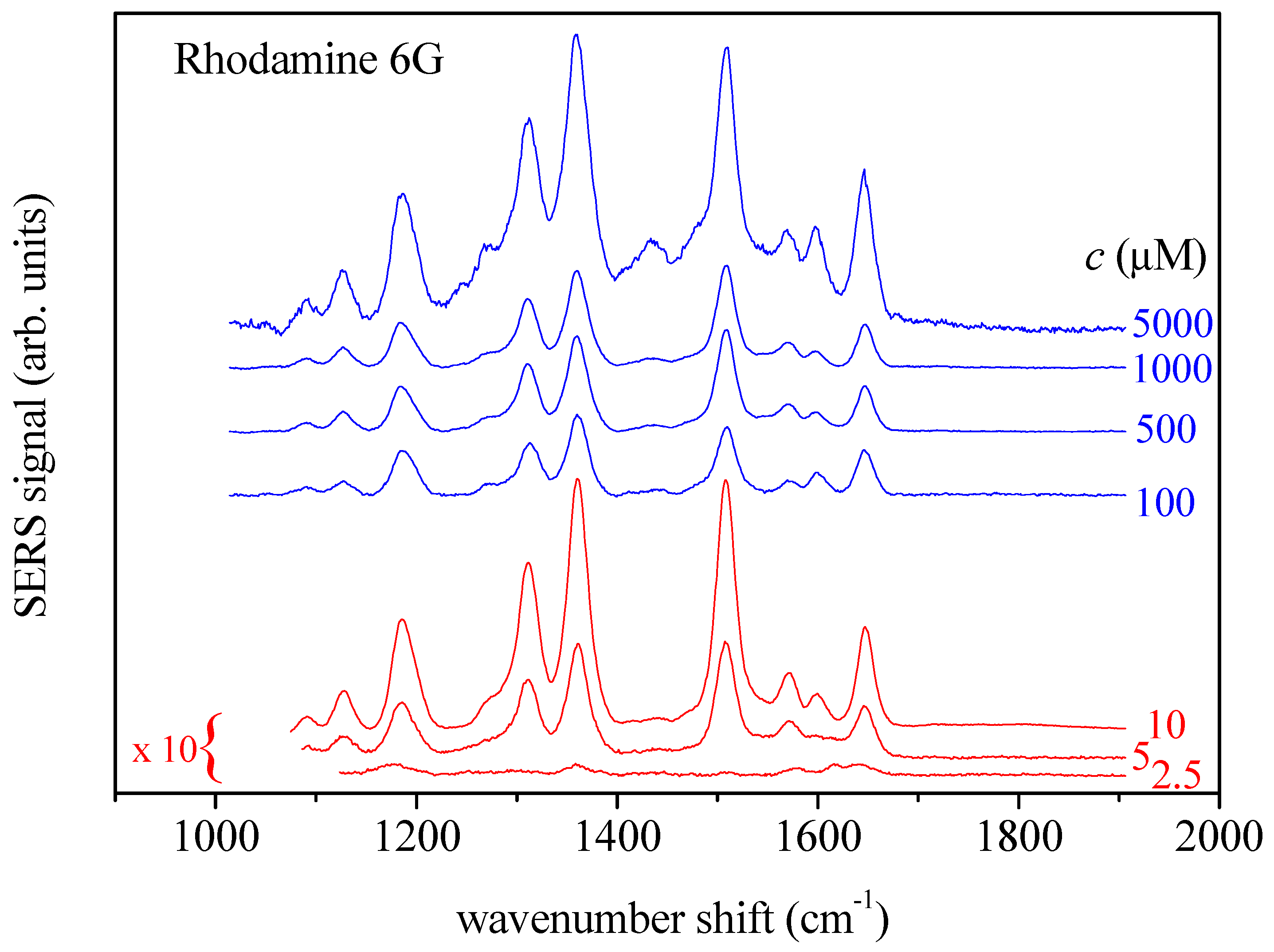
2.1. Rhodamine 6G Solutions
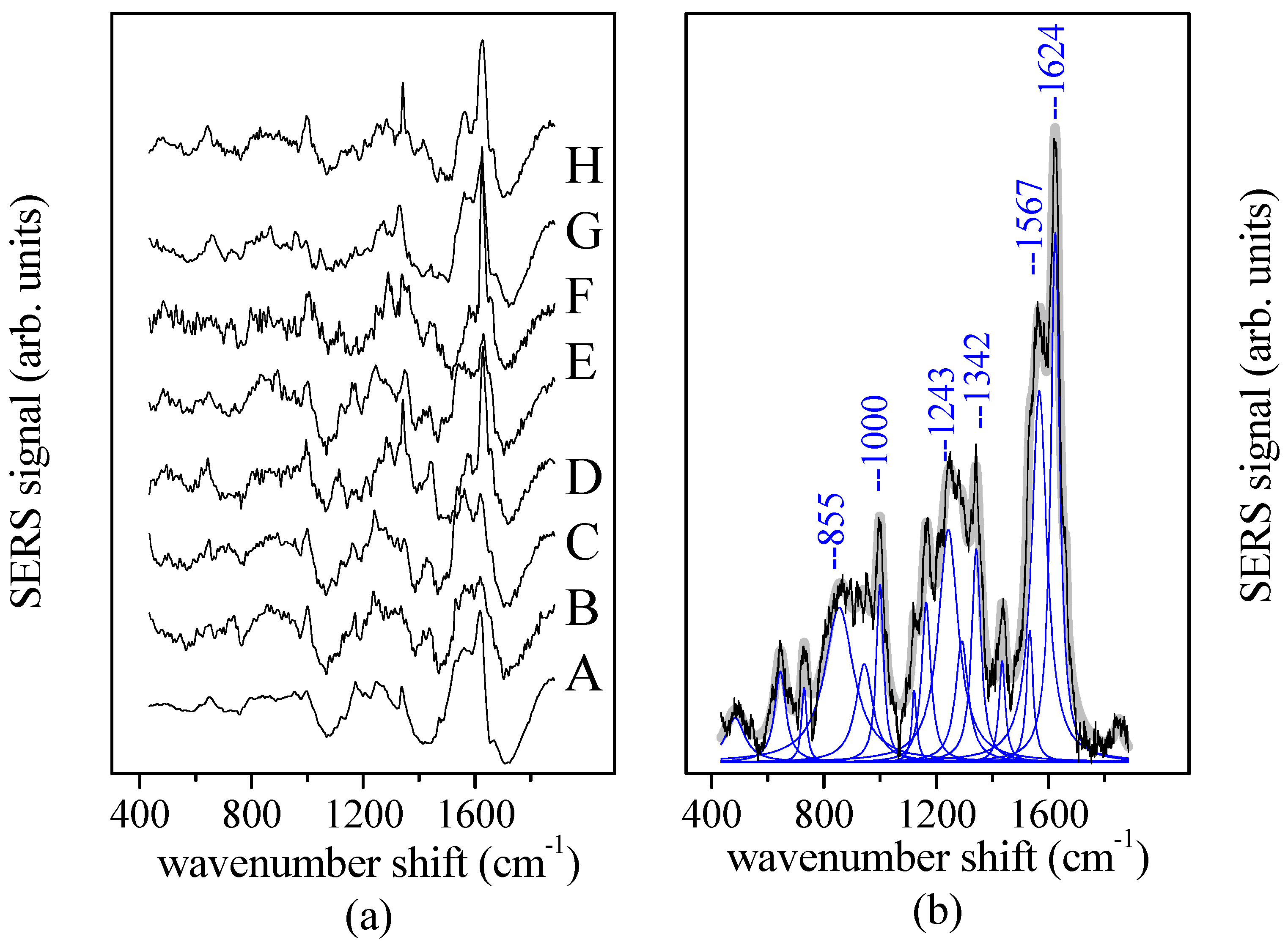
2.2. Tear Collection
2.3. SERS Measurements
2.4. Spectral Data Analysis
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef]
- Börger, M.; Funke, S.; Bähr, M.; Grus, F.; Lingor, P. Biomarker sources for Parkinson’s disease: Time to shed tears? Basal Ganglia 2015, 5, 63–69. [Google Scholar] [CrossRef]
- D’apuzzo, F.; Perillo, L.; Delfino, I.; Portaccio, M.; Lepore, M.; Camerlingo, C. Monitoring early phases of orthodontic treatment by means of Raman spectroscopies. J. Biomed. Opt. 2017, 22, 115001. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Zenone, F.; Perna, G.; Capozzi, V.; Cirillo, N.; Gaeta, G.M.; Lepore, M. An investigation on micro-Raman spectra and wavelet data analysis for Pemphigus Vulgaris follow-up monitoring. Sensors 2008, 8, 3656–3664. [Google Scholar] [CrossRef] [PubMed]
- Gąsior-Głogowska, M.; Komorowska, M.; Hanuza, J.; Mączka, M.; Zając, A.; Ptak, M.; Będziński, R.; Kobielarz, M.; Maksymowicz, K.; Kuropka, P.; et al. FT-Raman spectroscopic study of human skin subjected to uniaxial stress. J. Mech. Behav. Biomed. Mater. 2013, 18, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Reichebacher, M.; Popp, J. Challenges in molecular structure determination. In Vibrational Spectroscopy XX; Springer: Berlin/Heidelberg, Germany, 2013; pp. 63–143. [Google Scholar]
- Filik, J.; Stone, N. Analysis of human tear fluid by Raman spectroscopy. Anal. Chim. Acta 2008, 616, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Filik, J.; Stone, N. Investigation into the protein composition of human tear fluid using centrifugal filters and drop coating deposition Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 218–224. [Google Scholar] [CrossRef]
- Kuo, M.-T.; Lin, C.-C.; Liu, H.-Y.; Chang, H.-C. Tear Analytical model based on Raman microspectroscopy for investigation of infectious diseases of the ocular surface. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4942–4950. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zheng, X.S.; Zong, C.; Li, M.H.; Zhang, L.Y.; Li, W.; Ren, B. Drop-coating deposition and surface-enhanced Raman spectroscopies (DCDRS and SERS) provide complementary information of whole human tears. J. Raman Spectrosc. 2014, 45, 565–573. [Google Scholar] [CrossRef]
- Kim, W.-S.; Shin, J.-H.; Park, H.-K.; Choi, S. A low-cost, monometallic, surface-enhanced Raman scattering-functionalized paper platform for spot-on bioassays. Sens. Actuators B Chem. 2016, 222, 1112–1118. [Google Scholar] [CrossRef]
- Choi, S.; Moon, S.W.; Shin, J.-H.; Park, H.-K.; Jin, K.-H. Label-free biochemical method for early detection of Adenoviral conjuctivitis using human tear biofluids. Anal. Chem. 2014, 86, 11093–11099. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Particle size and sol stability in metal colloids. Colloid Polym. Sci. 1972, 250, 736–741. [Google Scholar] [CrossRef]
- Camerlingo, C.; Portaccio, M.; Tatè, R.; Lepore, M.; Delfino, I. Fructose and pectin detection in fruit-based food products by Surface-Enhanced Raman Spectroscopy. Sensors 2017, 17, 839. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Portaccio, M.; Tatè, R.; Lepore, M.; Delfino, I. Surface-Enhanced Raman Spectroscopy study of commercial Fruit Juices. Proceedings 2017, 1, 25. [Google Scholar]
- Camerlingo, C.; Zenone, F.; Gaeta, G.M.; Riccio, R.; Lepore, M. Wavelet data processing of micro-Raman spectra of biological samples. Meas. Sci. Technol. 2016, 17, 298–303. [Google Scholar] [CrossRef]
- Ameer, F.S.; Pittman, C.U., Jr.; Zhang, D. Quantification of resonance Raman enhancement factors for Rhodamine 6G (R6G) in Water and on Gold and Silver Nanoparticles: Implications for Single-Molecule R6G SERS. J. Phys. Chem. C 2013, 117, 27096–27104. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Gühlke, M.; Kneipp, J. Surface-enhanced hyper-Raman spectra of adenine, Guanine, Cytosine, Thymine, and Uracil. J. Phys. Chem. 2016, 120, 15415–15423. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Fredericks, P.M. Surface-enhanced Raman spectroscopy of amino acids adsorbed on an electrochemically prepared silver surface. Spectrochim. Acta Part A 1999, 55, 1641–1660. [Google Scholar] [CrossRef]
- Hu, J.; Sheng, R.S.; Xu, Z.S.; Zeng, Y. Surface enhanced Raman spectroscopy of lysozyme. Spectrochim. Acta 1995, 51A, 1087–1096. [Google Scholar] [CrossRef]
- Manciu, F.S.; Ciubuc, J.D.; Sundin, E.M.; Qiu, C.; Bennet, K.E. Analysis of Serotin molecules on silver nanocolloids—A Raman computational and experimental study. Sensors 2017, 17, 1741. [Google Scholar] [CrossRef] [PubMed]
| Identifier | Birth Year | Sex |
|---|---|---|
| A | 1985 | f |
| B | 1980 | f |
| C | 1972 | f |
| D | 1956 | f |
| E | 1952 | m |
| F | 1948 | m |
| G | 1943 | f |
| D | 1933 | m |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camerlingo, C.; Lisitskiy, M.; Lepore, M.; Portaccio, M.; Montorio, D.; Prete, S.D.; Cennamo, G. A Preliminary Investigation on Human Tears by Means of Surface Enhanced Raman Spectroscopy. Proceedings 2019, 4, 18. https://doi.org/10.3390/ecsa-5-05737
Camerlingo C, Lisitskiy M, Lepore M, Portaccio M, Montorio D, Prete SD, Cennamo G. A Preliminary Investigation on Human Tears by Means of Surface Enhanced Raman Spectroscopy. Proceedings. 2019; 4(1):18. https://doi.org/10.3390/ecsa-5-05737
Chicago/Turabian StyleCamerlingo, Carlo, Mikhail Lisitskiy, Maria Lepore, Marianna Portaccio, Daniela Montorio, Salvatore Del Prete, and Gilda Cennamo. 2019. "A Preliminary Investigation on Human Tears by Means of Surface Enhanced Raman Spectroscopy" Proceedings 4, no. 1: 18. https://doi.org/10.3390/ecsa-5-05737
APA StyleCamerlingo, C., Lisitskiy, M., Lepore, M., Portaccio, M., Montorio, D., Prete, S. D., & Cennamo, G. (2019). A Preliminary Investigation on Human Tears by Means of Surface Enhanced Raman Spectroscopy. Proceedings, 4(1), 18. https://doi.org/10.3390/ecsa-5-05737







