The underlying idea of any field-based 3-D QSAR is that differences in a target propriety, e.g., biological activity, are often closely related to equivalent changes in shapes and intensities of noncovalent calculated interaction surrounding the molecules (also called molecular interaction fields, MIFs). This concept was introduced in 1988 by Cramer et al. with the well-known Comparative Molecular Field Analysis (CoMFA). The procedure to build a MIF-based 3-D QSAR model involves the following steps: training-set selection, alignment of molecules’ conformations, MIF calculation, statistical model definition, model validation, and graphical interpretation. To perform all the steps, any user is asked to install specialized software, either costly or even open source, which require the user to have informatics skills. Herein is presented the very first series of web applications by which 3-D QSAR models can be easily built and graphically analyzed. The following web applications are available: (1) Py-MolEdit that enables the compilation of the data set through either uploading a list of molecules in any openbabel recognized format or by direct drawing through a java script molecular editor; (2) Py-ConfSerch that contains different conformational analysis engines to generate conformational ensembles for each dataset molecules; (3) Py-Align, through automatic molecular alignment software, leads to molecular alignment on up to 16 pre-defined different templates conformations or user selected ones; (4) Py-CoMFA that allows the building and validation of the 3-D QSAR model in the same fashion of the original CoMFA software (Figure 1). Different tools are available to inspect the models' results either numerically or graphically, all through a standard web browser and without the need to install any additional program. As it has been designed to be fully responsive, the www.3d-qsar.com portal can be used by means of any electronic device that can surf the internet.
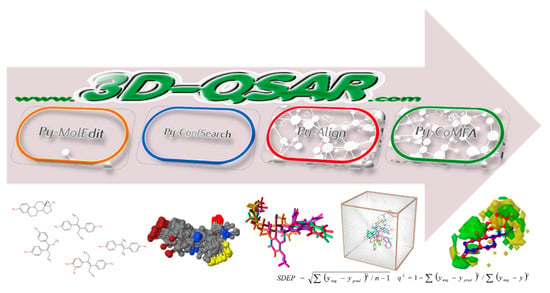
Figure 1.
3-D QSAR modeling workflow as implemented in www.3d-qsar.com.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).