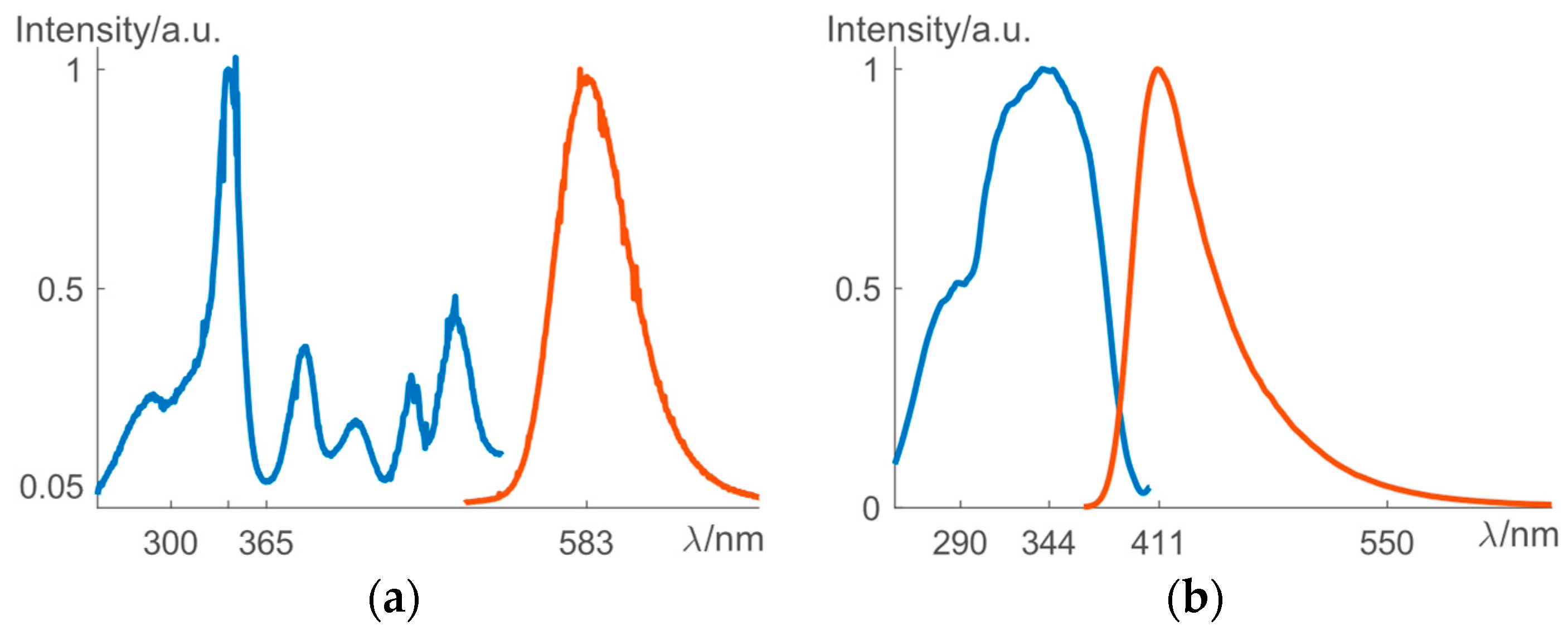
The solid-state synthesis yielded pure phases of the investigated ML materials (X-Ray diffraction patterns not shown here). As can be seen from
Figure 1a, ZnS:1%Mn
2+ can be excited efficiently by light of wavelength up to 500 nm, and its emission band is centered at 583 nm, which results in a yellow-orange color. (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+, 1%Ho
3+ can be excited by UV light from 250–400 nm and the emission band is centered at 411 nm, which corresponds to a blue color (
Figure 1b). Because of their non-centrosymmetric crystal structures, both phosphors show piezoelectricity, i.e., an internal electric field arises due to the application of stress. ZnS:1%Mn
2+ is very sensitive to friction and is applicable in dynamic pressure mapping, especially above 10 MPa [
7], but the response to extension was not reported yet, which could reveal interesting physics of ML.
3.1. Mechanoluminescence as a Visulization of Stress Fields
The tests on phosphor-embedded polymer discs (radius
R, thickness
l) display a clear ML pattern, as shown in
Figure 2a, whose asymmetry is due to the imperfect loading conditions. The theoretical stress field under a diametric loading pair
P (along
y, −
y orientations), is as follows [
8]:
and the Von Mises stress
σVM is defined as:
From
Figure 2b, the ML pattern agrees with the contour of
σVM, which indicates that the deformation energy plays a vital role in inducing ML. Actually,
σVM is directly related to the stress deviator,
sij =
σij − Σ
σii/3 through
σVM = (1.5
sij·
sij)
0.5, which is responsible for the change of shape of materials [
9]. On the other hand, the hydraulic stress Σ
σii/3 is responsible for the change of volume. Thus, it is possible that mechanical loads excite various defects in ML phosphors, such as point defects, dislocations, faults, etc., and then release electrons that were stored during the excitation by UV light.
It is interesting to note that (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+,1%Ho
3+ is also able to visualize the stress field produced by ultrasonic stimulation via acoustically induced piezoluminescence (APL), as shown in
Figure 2c. The ring and circle at the center of the image correspond to the stress field of ultrasonic irradiation. The asymmetry is due to the imperfection of the transducer. This effect lasted tens of seconds during the ultrasonic radiation, but disappeared in 1–2 s after the ultrasound was removed.
The visualization of stress fields heavily depends on the linearity between stress and ML. This linearity can be readily confirmed in a series of tensile experiments. It is worth noting that the afterglow should be subtracted from the observed emission profile, which is the compound of net ML and afterglow, as shown in
Figure 3a. For an extension from 0.00 to 0.60 mm at a rate of 1.2 mm/s, a net ML profile can be obtained, shown in
Figure 3b, and the linear relationship between ML and load is clearly observed after a threshold around 2 MPa, as shown in the insect. A long tail up to tens of seconds prevails after the peak of ML, which is related to the combination of recombination with luminescent centers and re-trapping at defects of electrons liberated due to stress. The delay time between the end of the UV excitation and the application of the load influences the ML magnitude greatly. In
Figure 3c, the magnitude of the ML peak drops very quickly as the delay time is increased. However, the afterglow also decays with time, resulting in the maximum ML contrast, i.e., ML/afterglow (inset of
Figure 3c), after a delay of 90 s. Therefore, for visualizing stress distribution, it is important to incorporate a delay time in order to reach a good compromise between ML intensity and ML contrast.
3.2. Trap Properties as a Mean to Model Mecholuminescence
The periodic packing of atoms in crystals makes it possible to describe the energy of electrons with a certain wave vector k, i.e., E(k) in a band diagram. Here, the electrons of filled orbitals form the valence band, which is separated by the bandgap from the conduction band, where electrons can move freely in the entire crystal if they are excited above the band gap. However, doping with impurity ions such as Mn
2+ or Eu
2+ leads to additional energy levels inside the band gap. Special synthesis procedures (such as sintering in reducing gas, H
2) will create additional defects as well, which are able to donate/accept charge carriers to/from the crystal and even trap these charge carriers, for example in glow-in-the-dark phosphors. The concentration of traps n and the energy levels with respective to the conduction band minimum, i.e., the trap depth E
trap, play an important role. For example, electrons trapped at defects can escape at a rate p = s·exp(−E
trap/kT), contributing n∙p to the change of afterglow in many cases, in which k is the Boltzmann constant, s an attempt-to-escape frequency and T the temperature. Thermoluminescence (TL), i.e., luminescence intensity as a function of temperature while heating at a fixed rate (dT/dt = β), is a very useful tool to reveal the trap depths and trap concentration. In a TL profile, a distinct glow peak profile (at a certain temperature T
m) can correspond to a trap depth E
trap, and the area under the curve is proportional to the trap concentration. Various methods are available to calculate the trap depths, see [
10] for a review.
Among these methods, the initial rise method is generally applicable to extract trap depth and does not depend on the kinetics of the detrapping mechanism of electrons [
11]. It is assumed that, at the early rise of a TL peak, the rate of electrons escaping from a trap is negligible and the intensity of TL follows from
where k is the Boltzmann constant, T the temperature and C a constant.
For (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+, the introduction of 1%Ho
3+ lowered the height of the TL peak in
Figure 4a, which indicates a lower trap concentration. It also shifted the peak temperature T
m to higher values, and thus increased the trap depth from 0.623 eV (for (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+) to 0.638 eV as calculated from the fitting of the linear part (below 20% of the intensity at T
m) of the log(I
TL)-1/T plot in
Figure 4b. Interestingly, the TL of ZnS:1%Mn
2+ was negligible, under excitation by 302 nm (exciting ZnS host) or 365 nm (exciting mainly Mn
2+), which meant that the trap concentration was very low, or the trap depth was so small that all traps were already emptied before the TL started [
12]. It is notable that the ML of (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+, 1%Ho
3+ is higher than that of (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+. Furthermore, ZnS:1%Mn
2+ shows limited ML under tensile extension although its efficient ML due to friction, scratching or compression of single crystals have been reported [
13]. This is probably due to the very limited trap concentration in ZnS:1%Mn, which prohibited appreciable ML. Normally, ML is due to the release of trapped electrons. However, these trapped electrons may have a different trap depth distribution from the traps responsible for afterglow. For example, deeper traps can yield ML where afterglow is minimal (a delay of 290 s still can provide ML in
Figure 3c). Judging from
Figure 4a–c, it is concluded that the traps in (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+ responsible for ML are situated in the deeper part of the trap depth distribution. Therefore, the introduction of Ho
3+ enhances ML as it shifts the traps to larger depth although the trap concentration is only slightly decreased. The ML contrast was hereby improved from 0.15 (for (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+) to 0.28 (for (Ca
0.4Sr
0.6)Al
2Si
2O
8:1%Eu
2+,1%Ho
3+). The combination of a reduction of trap concentration and an increase of trap depth is responsible for the improvement.
This indicates that tuning the trap depths and trap concentration is a useful way to tailor the properties of ML according to specific applications such as stress visualization, APL, or ML displays. This is possible when the traps responsible for ML are already known for the studied materials.