Freeze Desalination as Point of Use Water Treatment Technology: A Case of Chromium (VI) Removal from Water †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Setup
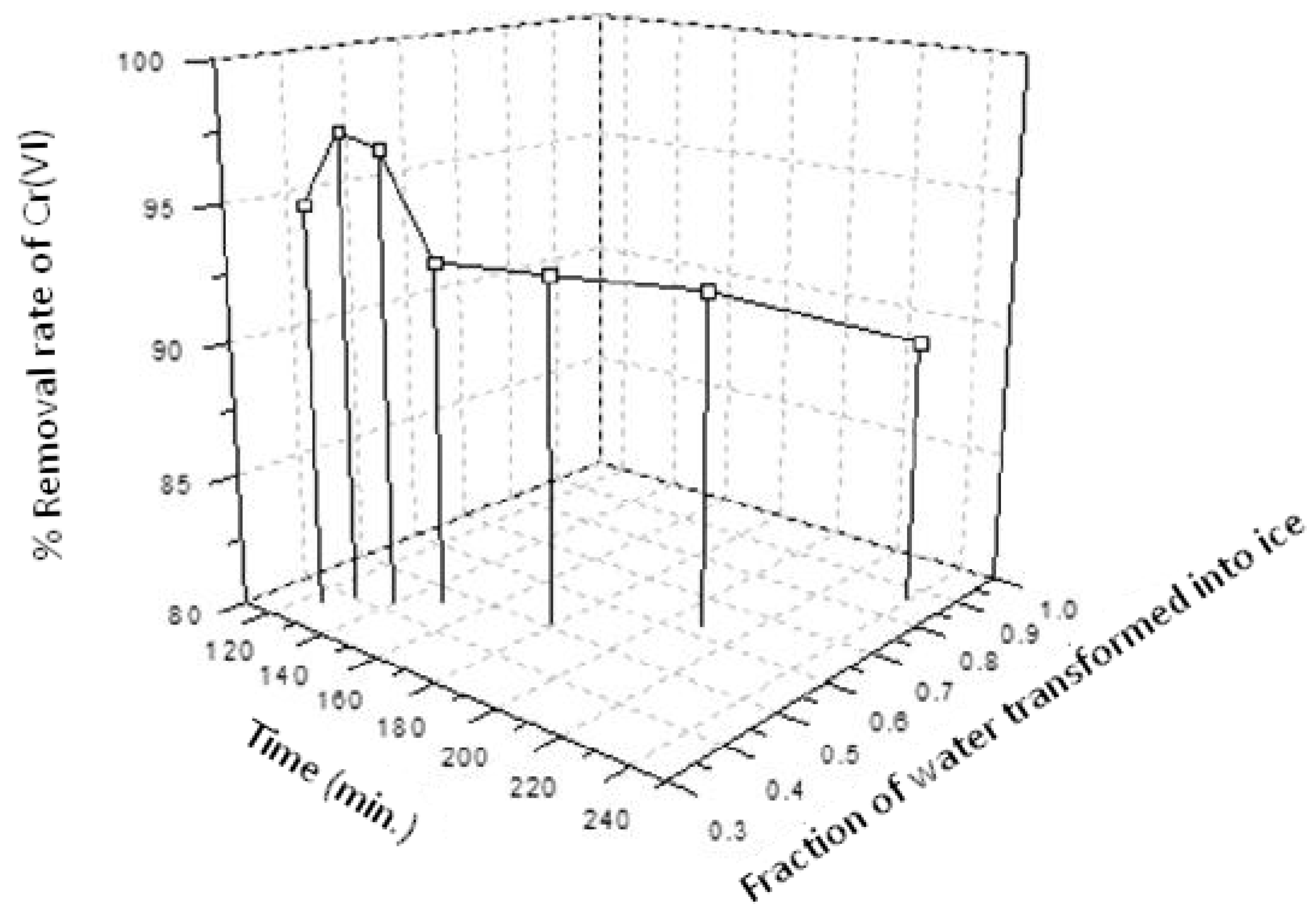
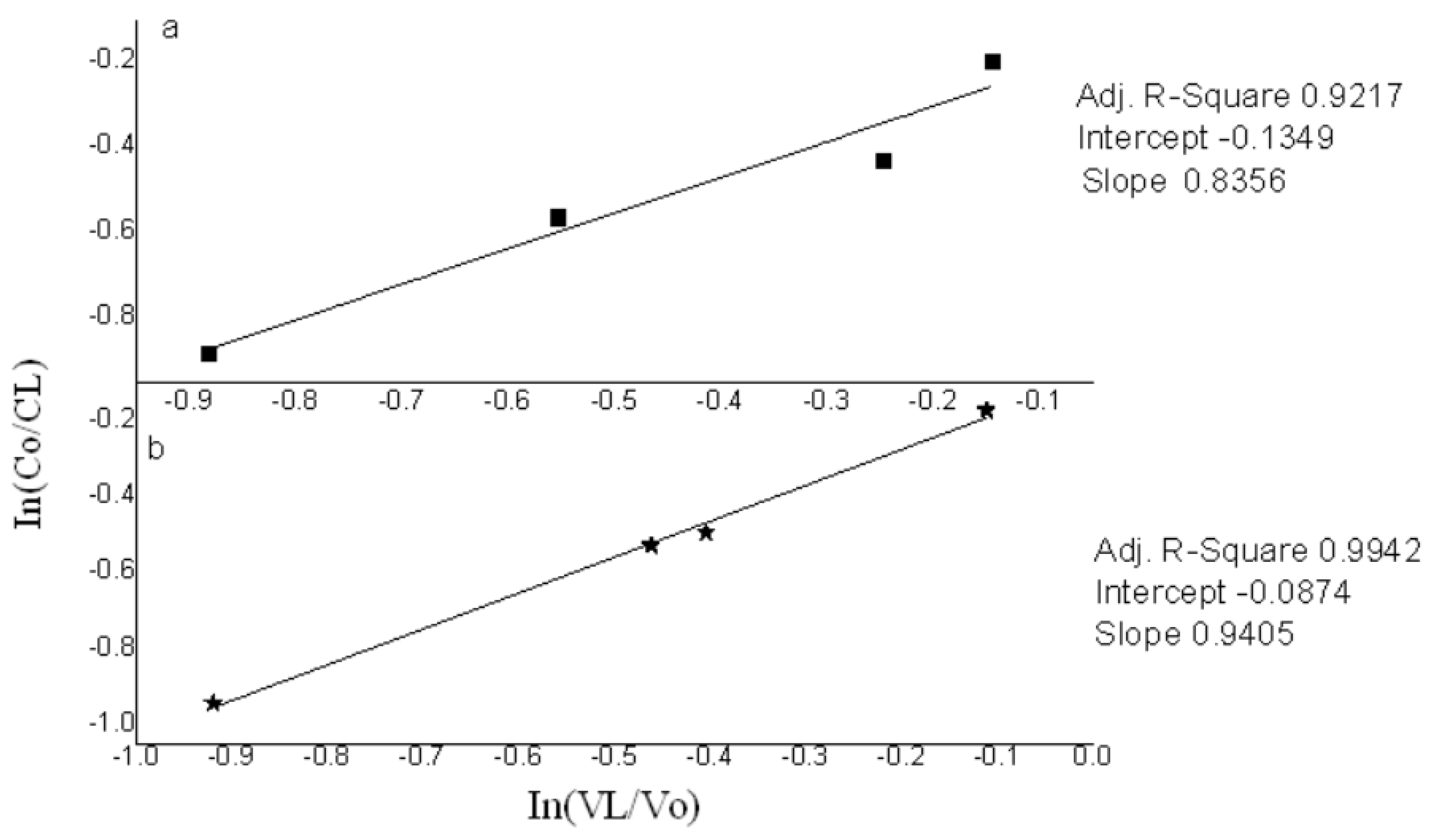
3. Results and Discussion
3.1. Deionized Water Spiked with Cr(VI)
3.2. Validating Cr(VI) Removal from Simulated Tap Water
3.3. Effect of Sodium Chloride Application
3.4. Energy Efficiency Views
4. Conclusions
Conflicts of Interest
References
- Alemayehu, T. Heavy metal concentration in the Urban Environment of Addis Ababa, Ethiopia. Soil Sediment Contam. 2006, 15, 591–602. [Google Scholar] [CrossRef]
- Mannucci, A.; Munz, G.; Mori, G.; Lubello, C. Anaerobic treatment of vegetable tannery wastewaters: A review. Desalination 2010, 264, 1–8. [Google Scholar] [CrossRef]
- Hoyer, P.B. (Ed.) Ovarian Toxicology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Zinabu, G.M.; Pearce, N.J. Concentrations of heavy metals and related trace elements in some Ethiopian rift-valley lakes and their in-flows. Hydrobiologia 2003, 492, 171–178. [Google Scholar] [CrossRef]
- Mengistie, E.; Ambelu, A.; Van Gerven, T.; Smets, I. Impact of Tannery Effluent on the Self-purification Capacity and Biodiversity Level of a River. Bull. Environ. Contam. Toxicol. 2016, 96, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Masresha, A.E.; Skipperud, L.; Rosseland, B.O.; Zinabu, G.M.; Meland, S.; Teien, H.C.; Salbu, B. Speciation of selected trace elements in three ethiopian rift valley lakes (koka, ziway, and awassa) and their major inflows. Sci. Total Environ. 2011, 409, 3955–3970. [Google Scholar] [CrossRef]
- Witt, K.L.; Stout, M.D.; Herbert, R.A.; Travlos, G.S.; Kissling, G.E.; Collins, B.J.; Hooth, M.J. Mechanistic insights from the NTP studies of chromium. Toxicol. Pathol. 2013, 41, 326–342. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Stanin, F.T. The Transport and Fate of Chromium (VI) in the Environment; Guertin, J., Jacobs, J.A., Avakian, C.P., Eds.; >Chromium (VI) Handbook; CRC Press: Boca Raton, FL, USA, 2005; pp. 165–199. [Google Scholar]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Yeon, K.H.; Moon, S.H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 2011, 196, 131–138. [Google Scholar] [CrossRef]
- Shi, L.N.; Zhang, X.; Chen, Z.L. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res. 2011, 45, 886–892. [Google Scholar] [CrossRef]
- Yari, A.R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Kalantari, R.R.; Yunesian, M. Application of a forward osmosis membrane in removal of chromium from aqueous solutions. Fresenius Environ. Bull. 2013, 22, 2319–2323. [Google Scholar]
- Scheumann, R.; Kraume, M. Influence of hydraulic retention time on the operation of a submerged membrane sequencing batch reactor (SM-SBR) for the treatment of greywater. Desalination 2009, 246, 444–451. [Google Scholar] [CrossRef]
- Das, C.; Patel, P.; De, S.; DasGupta, S. Treatment of tanning effluent using nanofiltration followed by reverse osmosis. Sep. Purif. Technol. 2006, 50, 291–299. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mahvi, A.H.; Nasseri, S.; Yunesian, M. Application of Freezing to the Desalination of Saline Water. Arab. J. Sci. Eng. 2011, 36, 1171–1177. [Google Scholar] [CrossRef]
- Lemmer, S.I.L.K.E.; Klomp, R.E.N.E.; Ruemekorf, R.; Scholz, R.E.I.N.H.A.R.D. Preconcentration of Wastewater through the Niro Freeze Concentration Process. Chem. Eng. Technol. 2001, 24, 485–488. [Google Scholar] [CrossRef]
- Gao, W.; Shao, Y. Freeze concentration for removal of pharmaceutically active compounds in water. Desalination 2009, 249, 398–402. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, L. Freezing desalination process. Therm. Desalin. Process. 2010, 2. [Google Scholar]
- Williams, P.M.; Ahmad, M.; Connolly, B.S.; Oatley-Radcliffe, D.L. Oatley-Radcliffe, Technology for freeze concentration in the desalination industry. Desalination 2015, 356, 314–327. [Google Scholar] [CrossRef]
- Lorain, O.; Thiebaud, P.; Badorc, E.; Aurelle, Y. Potential of freezing in wastewater treatment: Soluble pollutant applications. Water Res. 2001, 35, 541–547. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Guo, J.; Zhang, X. Application of freeze concentration for fluoride removal from water solution. J. Water Process Eng. 2017, 19, 260–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Anim-Danso, E.; Bekele, S.; Dhinojwala, A. Effect of Surface Energy on Freezing Temperature of Water. ACS Appl. Mater. Interfaces 2016, 8, 17583–17590. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zuo, J.; Lu, K.J.; Chung, T.S. Freeze desalination of seawater using LNG cold energy. Water Res. 2016, 102, 282–293. [Google Scholar] [CrossRef]
- Melak, F.; Du Laing, G.; Ambelu, A. Alemayehu, Application of freeze desalination for chromium (VI) removal from water. DES. 2016, 377, 23–27. [Google Scholar] [CrossRef]
- Hao, P.; Lv, C.; Zhang, X. Freezing of sessile water droplets on surfaces with various roughness and wettability. Appl. Phys. Lett. 2014, 104, 161–169. [Google Scholar] [CrossRef]
- Liu, L.; Miyawaki, O.; Nakamura, K. Progressive Freeze-Concentration of Model Liquid Food. Food Sci. Technol. Int. Tokyo 1997, 3, 348–352. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.N.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Feng, B.; Xu, K.; Huang, A. Synthesis of graphene oxide/polyimide mixed matrix membranes for desalination. RSC Adv. 2017, 7, 2211–2217. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Conway, B.E.; Ayranci, E. Effective Ionic Radii and Hydration Volumes for Evaluation of Solution Properties and Ionic Adsorption. J. Solution Chem. 1999, 28, 163–192. [Google Scholar] [CrossRef]
- Hummer, G.; Pratt, L.R.; Garcıa, A.E. Free Energy of Ionic Hydration. J. Phys. Chem. 1996, 100, 1206–1215. [Google Scholar] [CrossRef]
- Badawy, S.M. Laboratory freezing desalination of seawater. Desalin. Water Treat. 2015, 57, 11040–11047. [Google Scholar] [CrossRef]
- Mel’nichenko, N.A.; Slobodyuk, A.B. Nuclear magnetic resonance study of sea-water freezing mechanisms: Temperature dependence of relative brine content in sea ice. J. Glaciol. 2013, 59, 711–718. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ahmed, M.; Chen, X.D. Freezing-Melting Process and Desalination: I. Review of the State-of-the-Art. Sep. Purif. Rev. 2006, 35, 59–96. [Google Scholar] [CrossRef]
- Attia, A.A. New proposed system for freeze water desalination using auto reversed R-22 vapor compression heat pump. Desalination 2010, 254, 179–184. [Google Scholar] [CrossRef]
- Lara, J.R.; Noyes, G.; Holtzapple, M.T. An investigation of high operating temperatures in mechanical vapor-compression desalination. Desalination 2008, 227, 217–232. [Google Scholar] [CrossRef]
- Rice, W.; Chau, D.S.C. Freeze desalination using hydraulic refrigerant compressors. Desalination 1997, 109, 157–164. [Google Scholar] [CrossRef]
- Lin, W.; Huang, M.; Gu, A. A seawater freeze desalination prototype system utilizing LNG cold energy. Int. J. Hydrogen Energy 2017, 42, 18691–18698. [Google Scholar] [CrossRef]
- Jiang, X.; Cheng, Z.; Ma, W.; Gao, Z.; Ma, X.; Wang, R. Removal of Ammonia from Wastewater by Natural Freezing Method. In Proceedings of the International Conference on Chemical, Material and Food Engineering, Kunming, China, 25–26 July 2015; pp. 174–177. [Google Scholar]
- Zhou, Y.; Tol, R.S. Evaluating the costs of desalination and water transport. Water Resour. Res. 2005, 41, 1–10. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef]
- Baayyad, I.; Semlali Aouragh Hassani, N.; Bounahmidi, T. Evaluation of the energy consumption of industrial hybrid seawater desalination process combining freezing system and reverse osmosis. Desalin. Water Treat. 2014, 56, 2593–2601. [Google Scholar] [CrossRef]
- Ettouney, H. Conventional Thermal Process, Seawater Desalination, Conventional and Renewable Energy Processes; Springer: Berlin, Germany, 2009; pp. 17–40. [Google Scholar]


| Parameters | Melted Ice | Melted Ice | Simulated | Deionized |
|---|---|---|---|---|
| obtained from | obtained from | tap water | Water | |
| Simulated | frozen deionized | Spiked | ||
| tap water | water spiked | with Cr | ||
| with Cr | ||||
| Conductivity (μS/cm) | 46.8 | 2.6 | 99.6 | 2.5 |
| pH | 7.4 | 6.5 | 7.9 | 6.4 |
| DO (mg/L) | 6.8 | 6.83 | 6.81 | 6.9 |
| Ca2+ (mg/L) | 28.5 | 58.6 | ||
| Mg2+ (mg/L) | 14.4 | 29.3 | ||
| Na+ (mg/L) | 32.4 | 92.67 | ||
| K+ (mg/L) | 5.59 | 19.98 | ||
| Cr+6 (mg/L) | 1.75 | 0.16 | 5 | 5 |
| 3.54 | 0.41 | 10 | 10 | |
| 14.1 | 1.28 | 40 | 40 | |
| 31.2 | 6.4 | 80 | 80 | |
| 41 | 9 | 100 | 100 | |
| 100 | 30.3 | 200 | 200 | |
| HCO3− (mg/) | NM | 470 | ||
| SO42− (mg/L) | NM | 60 |
| Methods | Total Energy kWh/m3 | Total Average Costs | Remark | References |
|---|---|---|---|---|
| Thermal: Multistage flash evaporation (MSF) | 10–16 | 1.0 $/m3 | Second largest installed desalting capacity in the world next to RO | [42] |
| Thermal: Multiple effect evaporation (ME) | 5.5–9 | about $1.0/m3 | [43] | |
| Membrane processes: Reverse osmosis (RO) | 6.95 | less than $0.5/m3 | For seawater | [44,45] |
| Electrodialysis (ED) | less than $1.0/m3 | For seawater | [42] | |
| about $0.6/m3 | For brackish water | |||
| Hybrid method: Coupling freezing and reverse osmosis (RO) | 5.17 | For seawater | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melak, F.; Ambelu, A.; Laing, G.D.; Alemayehu, E. Freeze Desalination as Point of Use Water Treatment Technology: A Case of Chromium (VI) Removal from Water. Proceedings 2018, 2, 173. https://doi.org/10.3390/ecws-2-04942
Melak F, Ambelu A, Laing GD, Alemayehu E. Freeze Desalination as Point of Use Water Treatment Technology: A Case of Chromium (VI) Removal from Water. Proceedings. 2018; 2(5):173. https://doi.org/10.3390/ecws-2-04942
Chicago/Turabian StyleMelak, Fekadu, Argaw Ambelu, Gijs Du Laing, and Esayas Alemayehu. 2018. "Freeze Desalination as Point of Use Water Treatment Technology: A Case of Chromium (VI) Removal from Water" Proceedings 2, no. 5: 173. https://doi.org/10.3390/ecws-2-04942
APA StyleMelak, F., Ambelu, A., Laing, G. D., & Alemayehu, E. (2018). Freeze Desalination as Point of Use Water Treatment Technology: A Case of Chromium (VI) Removal from Water. Proceedings, 2(5), 173. https://doi.org/10.3390/ecws-2-04942





