Abstract
Prostate cancer cells need androgens to grow and maintain like normal prostate cells, both utilize that Androgen Receptor (AR) function. Androgen receptor (AR) is expressed throughout the prostate cancer progression plays a critical role as a transcription factor in castration-dependent stages of disease. AR also interacts to many cellular proteins, including p53, to regulate apoptosis. Further, as the stabilization of p53 protein triggers apoptosis, p53 interacting small molecules such as Nutlin3a, are interpreted as cancer therapeutics. In this study, to find out how Nutlin3a-mediated p53 stabilization effect on AR signaling. Here, we investigated the dynamics of p53 binding to transcriptional targets of AR, and further investigated the variations of AR intracellular localization as well as transactivation in the presence of Nutlin3a. To do this, the changes in AR transactivation were investigated via luciferase reporter assay, which was performed by treating LNCaPs with different doses of Nutlin3a and resulted that transactivation was suppressed by Nutlin3a in a dose dependent manner. AR transactivation and sub-cellular localization were also studied by immunofluorescence assay and found that cytoplasmic-nuclear fractionation-coupled western blot analysis showed that Nutlin3a inhibits AR phosphorylation and nuclear translocation regardless of androgens.
1. Introduction
Androgen is a key regulator of male sexual differentiation as well as prostate development and carcinogenesis. Androgen-regulated gene expression is mediated by the action of androgen receptor (AR), which is a member of nuclear receptor superfamily that functions as a ligand-dependent transcription factor [1]. AR regulates the expression of target genes by binding to androgen response elements (AREs) in the genome, or by interacting with other transcription factors bound to their specific recognition sites [2]. The probasin gene is one such example; its promoter is used for reporter studies due to its AREs are specifically recognized by AR. The DNA-binding domain (DBD) of AR is linked to the ligand-binding domain (LBD) by a hinge region [3]. AR has become an attractive therapeutic target in aggressive and disseminated prostate cancer. Furthermore, Murine Double Minute2 (MDM2) can control expression levels and transcriptional activity of the AR [4,5] as well as p53, which is the negative regulator of MDM2, can also interact with the AR leading to repression of AR-mediated transcription [6,7]. In this study, we investigated the effect of p53 stabilization via Nutlin3a, which shows its activity by inhibiting MDM2 on AR transcriptional activity in LNCaP cells.
2. Materials and Methods
2.1. Cell Culture and Treatments
LNCaP cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained in RPMI 1640 medium with 10% FBS, l-glutamine, peniciline and streptomycin at 37 °C presence of 5% CO2. Nutlin treatments were performed with Nutlin3a (Sigma, Dorset, UK) 24 h at appropriate concentrations (2.5, 5 and 10 µM). Androgen treatments were performed with synthetic androgen R1881 (10 nM) for 6 h or 24 h.
2.2. Reporter Assay
1 × 104 LNCaP cells were seeded in 96 well culture plate and cultured at 37 °C for 48 h and transfected with PGL3Basic and pProbasin vectors for 24 h. Then the cells treated with Nutlin3a for 24 h and R1881 for 6 h and then washed with PBS and collected with passive lysis buffer. Luciferase activity was measured by using a dual-luciferase reporter activity kit (Promega, Mannheim, Germany) according to the manufacturer’s protocol.
2.3. Immunofluorescence Labeling and Microscopy
In order to investigate the intracellular localization of AR by immunofluorescence microscopy, cells grown on coverslips were treated nutlin3a. At the end of incubation, cells were rinsed with PBS, fixed with methanol for 30 min at −20 °C, permeabilized with triton X-100 for 5 min and blocked with 1% BSA in PBS for 5 min. Then primary antibody was added and incubated for 1 h at 37 °C rinsed with PBS for 4 times. Then secondary antibody incubation was performed at 37 °C for 20 min using Alexafluor (488 anti-rabbit) antibody and rinsed with PBS for 4 times. Finally, cells were washed and mounted on coverslips with 30% glycerol in PBS including 0.1 µg/mL DAPI and analyzed using DM4000B LED fluorescent microscope (Leica Camera AG, Wetzlar, Germany).
2.4. Sub-Cellular Fractionation
Briefly, in order to obtain cytoplasmic and nuclear fractions, 1 × 106 LNCaP cells were washed with PBS pelleted and resuspended in buffer. Then the subcellular fractionation was performed using differential centrifugation, which was described in previous publications [8].
3. Results
3.1. Nutlin3a Affects AR Transactivation
Previous studies have shown that MDM2 can control the expression levels and transcriptional activity of AR, where p53 can interact with AR and lead to suppression of AR-mediated transcription. Since the p53 and AR were known to share the same E3 ubiquitin ligase encoded by the MDM2 oncogene, effect of Nutlin3a on AR transactivation was investigated. Here, specifically pProbasin reporter plasmid was used for transfection into LNCaP cells in the absence and presence of androgens. The luciferase firefly activity was normalized according to the protein concentration. Then, the fold change in relative luciferase activity (RLA) was graphed by the ratio of androgen-treated cells to androgen-free cells in the pProbasin-luc transfected cells. The fold change for the control cells was 34.1, whereas 19.0, 12.8 and 10.5 for 2.5, 5 and 10 µM nutlin3a respectively (Figure 1A).
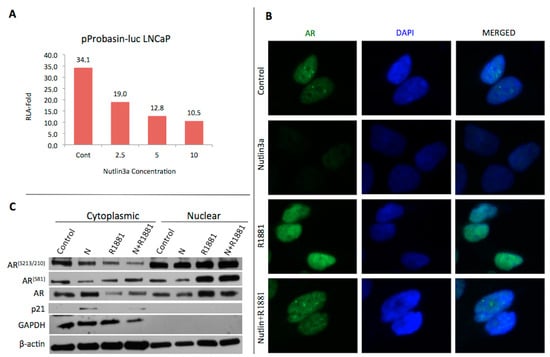
Figure 1.
(A) Dose dependent decrease in AR transcriptional activity is observed in nutlin3a treated cells. (B) AR localization in Nutlin3a (2.5 μM, 24 h) treated LNCaP cells with the presence (10 nM, 24 h) and absence of androgens. (C) Cytoplasmic-nuclear fraction. LNCaP cells were treated Nutlin3a (2.5 μM, 24 h) and/or androgens (10 nM, 24 h).
3.2. Nutlin3a Affects AR Translocation
LNCaP cells were treated with 2.5 µM Nutlin3a in the presence and absence of androgens for 24 h in order to investigate the intracellular localization of AR by immunofluorescence microscopy. We observed that AR was in both cytoplasm and nucleus in control cells, however significant reduction in nucleus localization was observed in nutlin3a-treated cells. When we investigated the AR expression in the presence of androgens, we observed a clear AR expression in nucleus, whereas reduced expression in nutlin3a treatment (Figure 1B). The data was confirmed in western blots showing that the ARS81 and ARS210 phosphorylations as well as total AR expression significantly decreased at cytoplasmic fractions after androgen and nutlin3a treatments. These data was also consistent with increased AR levels in nuclear fractions of untreated cells in comparison to androgen and nutlin3a-treated cells (Figure 1C).
4. Discussion
We found that AR transactivation was suppressed due to increasing concentrations of nutlins (Figure 1A). Since nutlin3a is known to activate the apoptotic pathway, we decided to use 2.5 µM of nutlin in subsequent experiments in order to understand the molecular mechanism due to increased concentration of cell death. We showed that the androgen receptor was decreased in the nucleus in nutlin3a-treated cells (Figure 1B). To understand the cause of this decrease, we performed cytoplasmic-nuclear fraction experiments. As a result, we found that AR decreased in nutlin3a treated cells in the nucleus and increased slightly in the cytoplasm. It shows that AR causes cytoplasmic retention by nutlin3a effect. Furthermore, the western blot results showed that the AR phosphorylations increase in the nucleus, due to the results of increase in AR stabilization, nutlin3a slightly decreased the AR translocation to nucleus. This result was concluded as MDM2 inhibitor nutlin3a affected androgen receptor-mediated transcription through increasing the cytoplasmic retention of AR itself.
Acknowledgments
This research was supported from TÜBİTAK by grant no 113S044 to KSK and BDB.
References
- Grossmann, M.E.; Huang, H.; Tindall, D.J. Androgen receptor signaling in androgen-refractory prostate cancer. J. Natl. Cancer Inst. 2011, 93, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Carroll, J.S.; Brown, M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol. Cell 2005, 19, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, E.; Verrijdt, G.; Peeters, B.; Verhoeven, G.; Rombauts, W.; Claessens, F. Differences in DNA binding characteristics of the androgen and glucocorticoid receptors can determine hormone-specific responses. J. Biol. Chem. 2000, 275, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Wang, L.; Hu, Y.C.; Altuwaijri, S.; Chang, C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002, 21, 4037–4048. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, L.; Logan, I.R.; Neal, D.E.; Robson, C.N. Regulation of androgen receptor and histone deacetylase 1 by Mdm2- mediated ubiquitylation. Nucleic Acids Res. 2005, 33, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Wasylyk, B. Physiological and pathological con- sequences of the interactions of the p53 tumor suppressor with the glucocorticoid, androgen, and estrogen receptors. Ann. N. Y. Acad. Sci. 2004, 1024, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Shenk, J.L.; Fisher, C.J.; Chen, S.Y.; Zhou, X.F.; Tillman, K.; Shemshedini, L. p53 represses androgen-induced transactivation of prostate- specific antigen by disrupting hAR amino- to carboxyl-terminal interaction. J. Biol. Chem. 2001, 276, 38472–38479. [Google Scholar] [CrossRef] [PubMed]
- Debelec-Butuner, B.; Alapinar, C.; Ertunc, N.; Gonen-Korkmaz, C.; Yorukoglu, K.; Korkmaz, K.S. TNFa-Mediated Loss of b-Catenin/E-Cadherin Association and Subsequent Increase in Cell Migration Is Partially Restored by NKX3.1 Expression in Prostate Cells. PLoS ONE 2014, 9, e109868. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).