Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
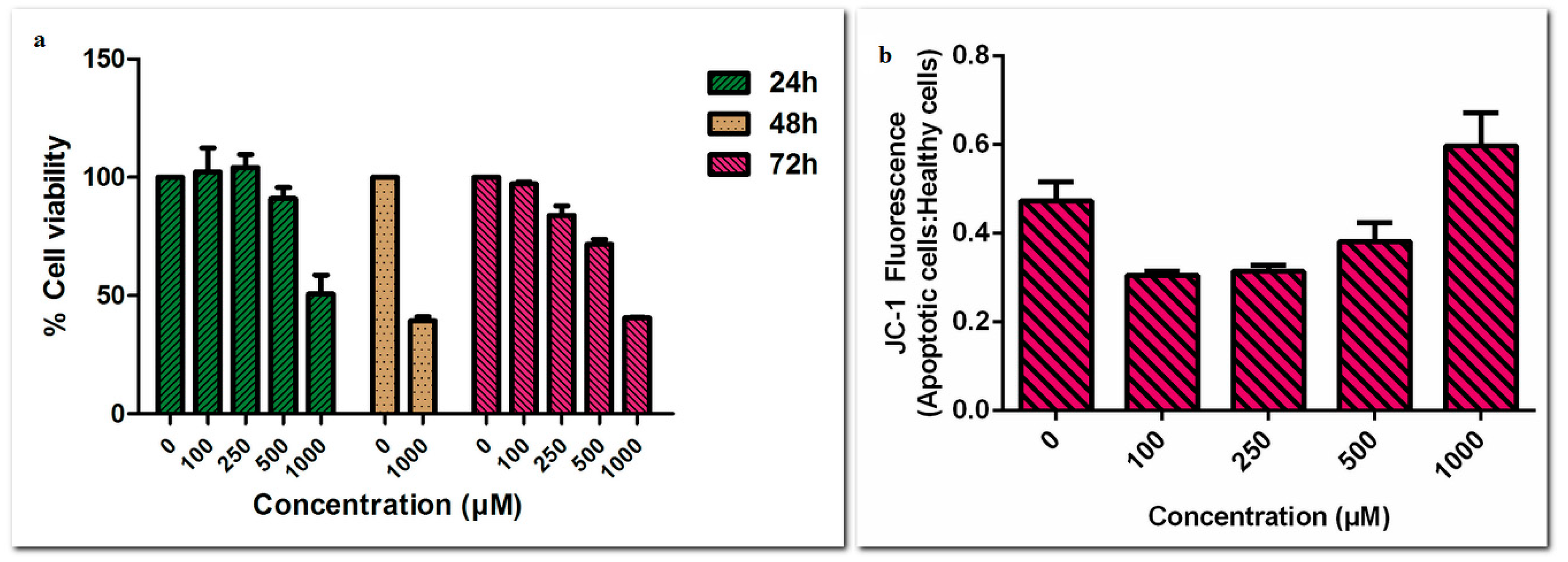
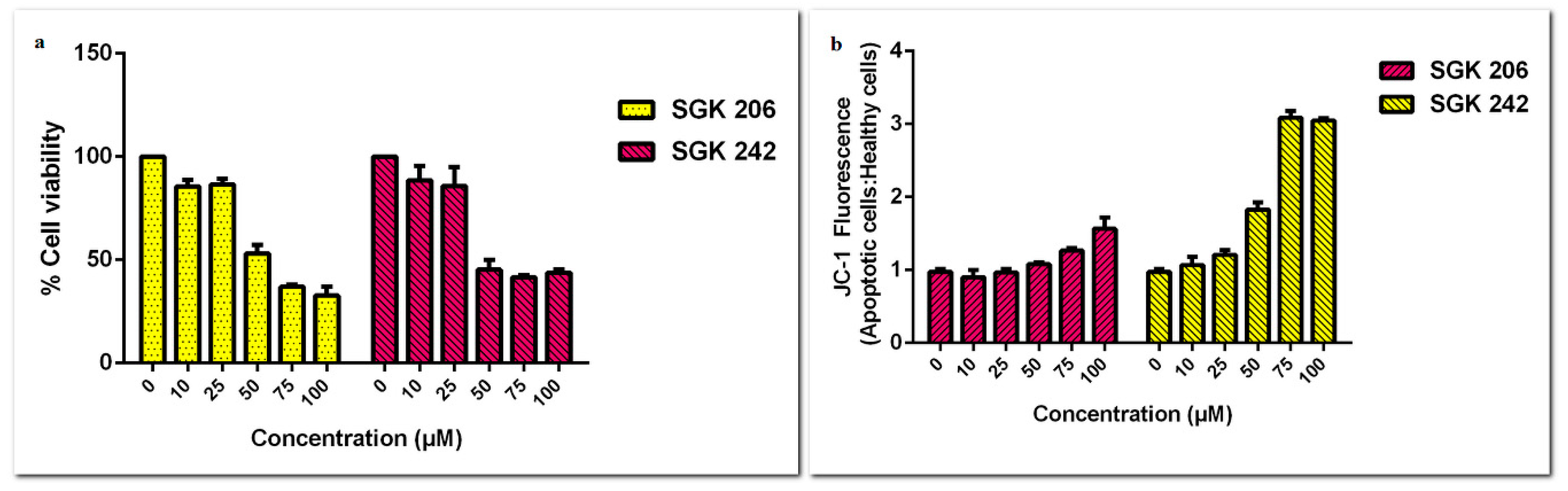
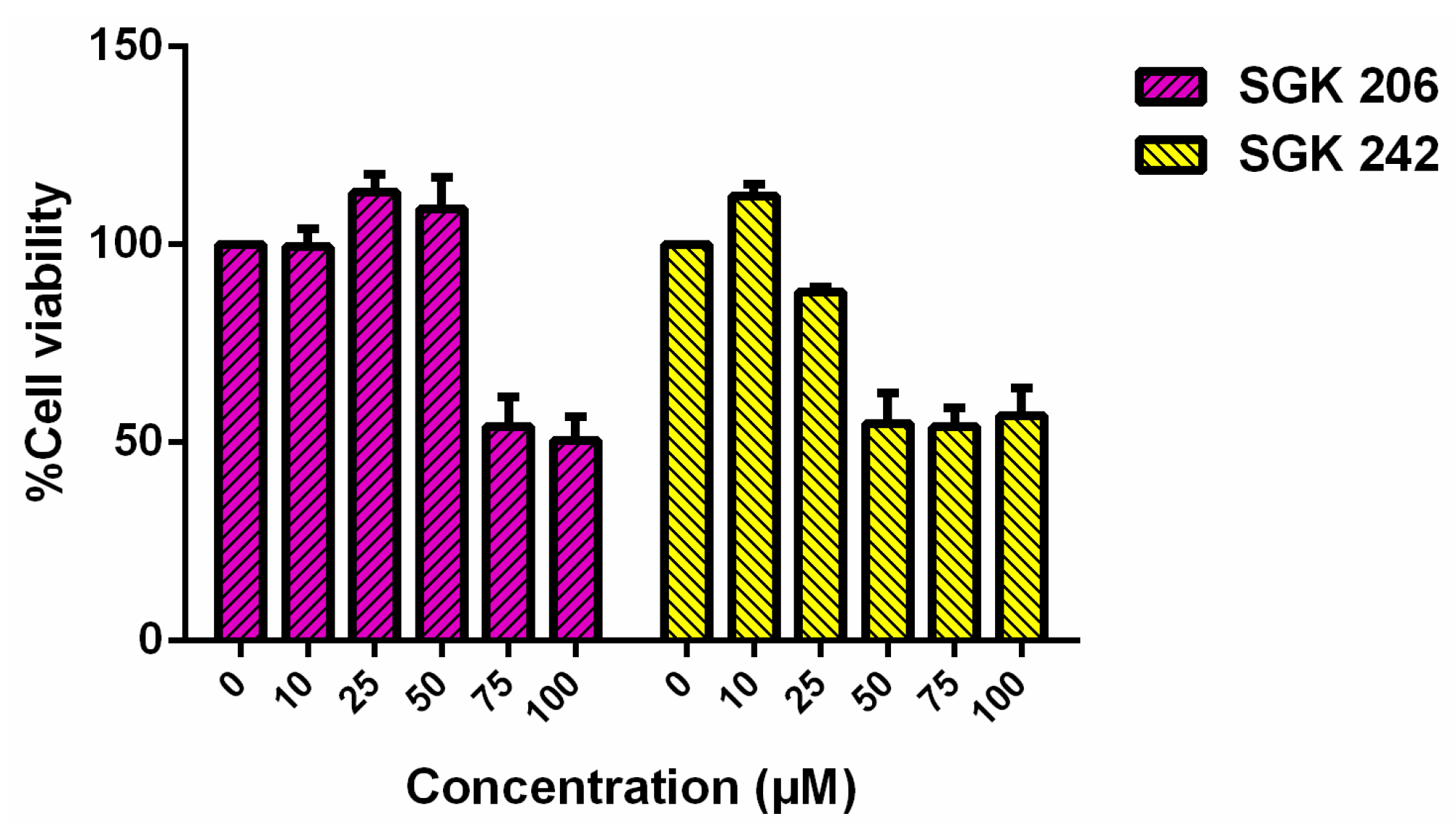
Acknowledgments
References
- Thun, M.J.; Henley, S.J.; Patrono, C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer Inst. 2002, 94, 252–266. [Google Scholar] [CrossRef]
- Hsi, L.C.; Joon Baek, S.; Eling, T.E. Lack of Cyclooxygenase-2 Activity in HT-29 Human Colorectal Carcinoma Cells. Exp. Cell Res. 2000, 256, 563–570. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakamura, S.; Shibata, K.; Sahara, N.; Shigeno, K.; Shinjo, K.; Naito, K.; Ohnishi, K. Etodolac inhibits EBER expression and induces Bcl-2-regulated apoptosis in Burkitt’s lymphoma cells. Eur. J. Haematol. 2005, 75, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Çıkla-Süzgün, P.; Özsavcı, D.; Bingöl-Özakpınar, Ö.; Şener, A.; Çevik, Ö.; Özbaş-Turan, S.; Akbuğa, J.; Şahin, F.; Küçükgüzel, Ş.G. Synthesis, cytotoxicity, and pro-apoptosis activity of etodolac hydrazide derivatives as anticancer agents. Arch. Pharm. Chem. Life Sci. 2013, 346, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Easmon, J.; Pürstinger, G.; Thies, K.S.; Heinisch, G.; Hofmann, J. Synthesis, structure-activity relationships, and antitumor studies of 2-benzoxazolyl hydrazones derived from alpha-(N)-acyl heteroaromatics. J. Med. Chem. 2006, 49, 6343–6350. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, Ş.G.; Koç, D.; Çıkla-Süzgün, P.; Özsavcı, D.; Bingöl-Özakpınar, Ö.; Mega-Tiber, P.; Orun, O.; Erzincan, P.; Sağ-Erdem, S.; Şahin, F. Synthesis of Tolmetin Hydrazide-Hydrazones and Discovery of a Potent Apoptosis Inducer in Colon Cancer Cells. Arch. Pharm. 2015, 348, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Cruz-Lopez, O.; Lopez Cara, C.; Carrion, M.D.; Brancale, A.; Hamel, E.; Chen, L.; Bortolozzi, R.; Basso, G.; et al. Synthesis and antitumor activity of 1,5-disubstituted 1,2,4-triazoles as cis-restricted combretastatin analogues. J. Med. Chem. 2010, 53, 4248–4258. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.; Park, H.J. Design, synthesis, and biological evaluation of novel 2-pyridinyl-[1,2,4]triazoles as inhibitors of transforming growth factor beta1 type 1 receptor. Bioorg. Med. Chem. 2004, 12, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevinç, S.K.; Orun, O.; Tiber, P.M.; Çıkla-Süzgün, P.; Küçükgüzel, Ş.G. Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines. Proceedings 2018, 2, 1573. https://doi.org/10.3390/proceedings2251573
Sevinç SK, Orun O, Tiber PM, Çıkla-Süzgün P, Küçükgüzel ŞG. Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines. Proceedings. 2018; 2(25):1573. https://doi.org/10.3390/proceedings2251573
Chicago/Turabian StyleSevinç, Sevgi Koçyiğit, Oya Orun, Pınar Mega Tiber, Pelin Çıkla-Süzgün, and Ş. Güniz Küçükgüzel. 2018. "Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines" Proceedings 2, no. 25: 1573. https://doi.org/10.3390/proceedings2251573
APA StyleSevinç, S. K., Orun, O., Tiber, P. M., Çıkla-Süzgün, P., & Küçükgüzel, Ş. G. (2018). Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines. Proceedings, 2(25), 1573. https://doi.org/10.3390/proceedings2251573



