Inflammatory Microenvironment-Mediated E-Cadherin Decrease Induces Migration in LNCaPs †
Abstract
:1. Introduction
2. Materials and Methods
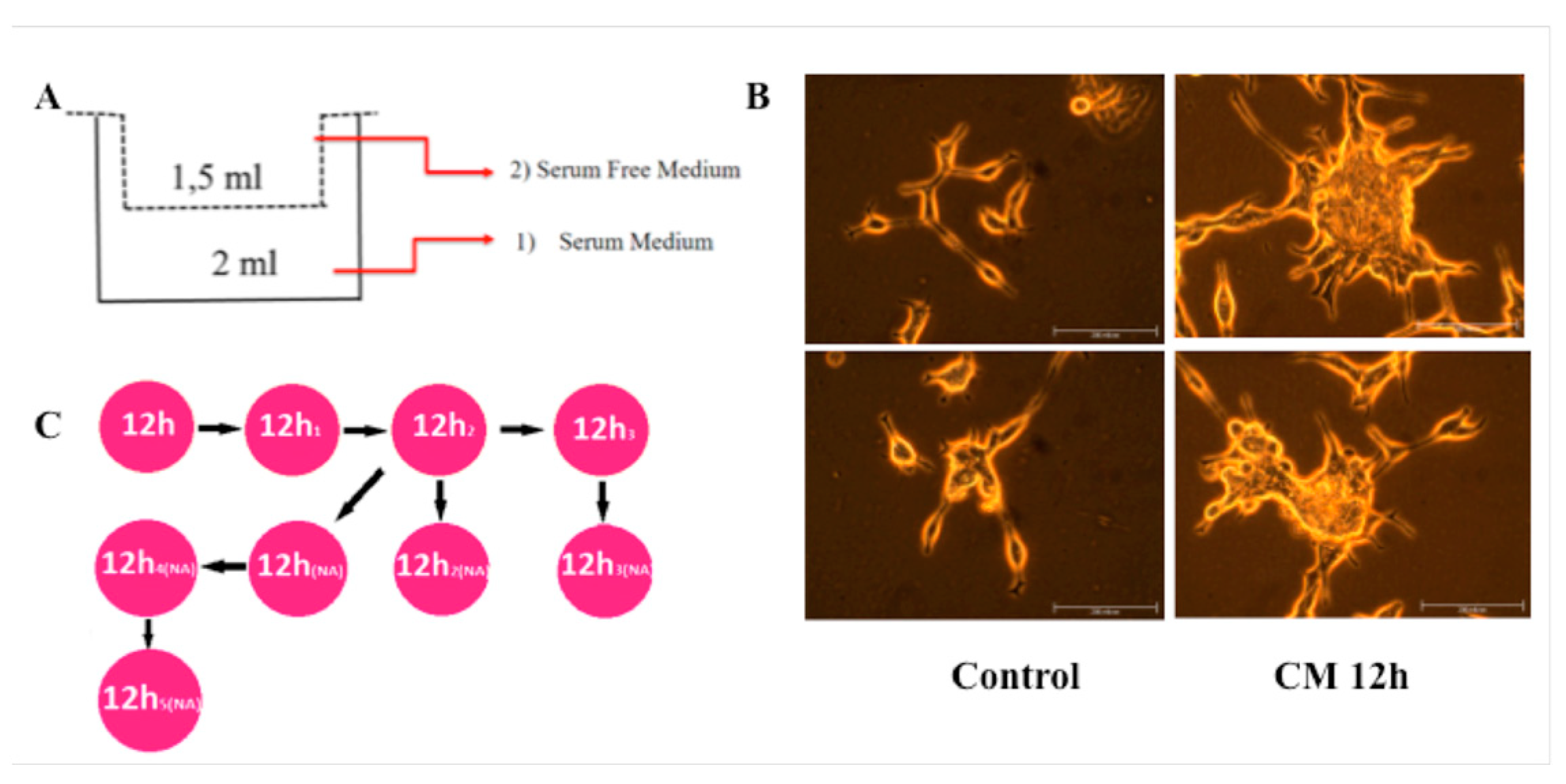
2.1. Macrophage Differentiation and Conditioned Media (CM) Collection
2.2. Measurement of Cytokines in the CM
2.3. Cell Culture and Treatments
2.4. Cell Migration Assay
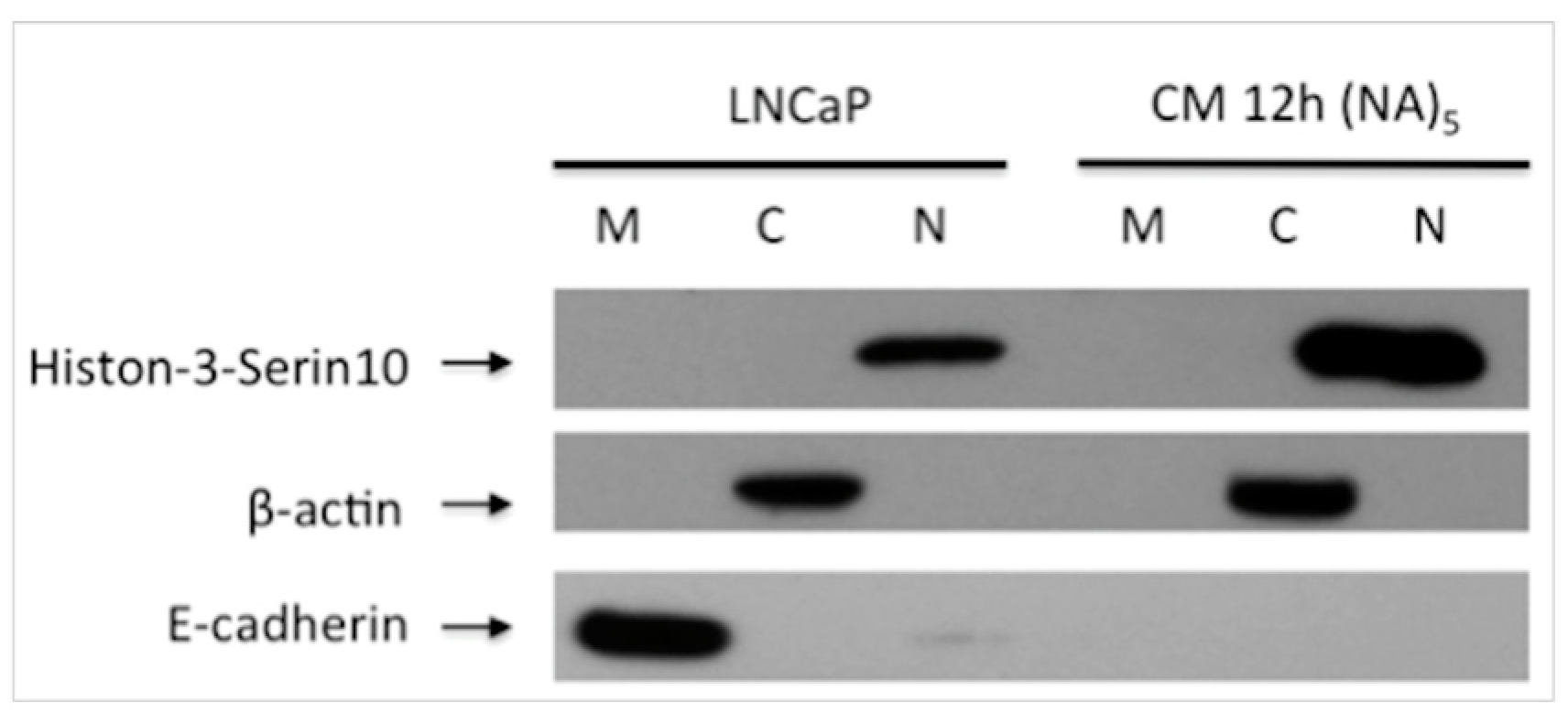
2.5. Sub-Cellular Fractionation
2.6. Western Blot Analysis
3. Results
3.1. CM Induces LNCaP Cell Migration
3.2. Decreased E-Cadherin Expression in Inflammation-Induced Migration of Prostate Cancer Cells
4. Discussion
Acknowledgments
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.K.; Mao, X.; Lu, Y.J. The complexity of prostate cancer: Genomic alterations and heterogeneity. Nat. Rev. Urol. 2012, 9, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Wang, H.; Poutanen, M.; Risbridger, G.P. Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic pre-malignancy. Am. J. Pathol. 2009, 175, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.S.; Han, I.H.; Sim, S.; Ahn, M.H.; Ryu, J.S. Proliferation of Prostate Stromal Cell Induced by Benign Prostatic Hyperplasia Epithelial Cell Stimulated with Trichomonas vaginalis via Crosstalk with Mast Cell. Prostate 2016, 76, 1431–1444. [Google Scholar] [CrossRef] [PubMed]
- Lo, U.G.; Lee, C.F.; Lee, M.S.; Hsieh, J.T. Review the Role and Mechanism of Epithelial-to-Mesenchymal Transition in Prostate Cancer Progression. Int. J. Mol. Sci. 2017, 18, 2079. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.M.; Kyprianou, N. Epithelial-mesenchymal transition (EMT) in prostate growth and tumor progression. Transl. Androl. Urol. 2013, 2, 202–211. [Google Scholar] [PubMed]
- Debelec-Butuner, B.; Alapinar, C.; Ertunc, N.; Gonen-Korkmaz, C.; Yörükoğlu, K.; Korkmaz, K.S. TNFa-Mediated Loss of b-Catenin/E-Cadherin Association and Subsequent Increase in Cell Migration Is Partially Restored by NKX3.1 Expression in Prostate Cells. PLoS ONE 2014, 9, e109868. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saydullaeva, I.; Bütüner, B.D.; Korkmaz, K.S. Inflammatory Microenvironment-Mediated E-Cadherin Decrease Induces Migration in LNCaPs. Proceedings 2018, 2, 1540. https://doi.org/10.3390/proceedings2251540
Saydullaeva I, Bütüner BD, Korkmaz KS. Inflammatory Microenvironment-Mediated E-Cadherin Decrease Induces Migration in LNCaPs. Proceedings. 2018; 2(25):1540. https://doi.org/10.3390/proceedings2251540
Chicago/Turabian StyleSaydullaeva, Iroda, Bilge Debeleç Bütüner, and Kemal Sami Korkmaz. 2018. "Inflammatory Microenvironment-Mediated E-Cadherin Decrease Induces Migration in LNCaPs" Proceedings 2, no. 25: 1540. https://doi.org/10.3390/proceedings2251540
APA StyleSaydullaeva, I., Bütüner, B. D., & Korkmaz, K. S. (2018). Inflammatory Microenvironment-Mediated E-Cadherin Decrease Induces Migration in LNCaPs. Proceedings, 2(25), 1540. https://doi.org/10.3390/proceedings2251540



