Abstract
In the study, we aimed to show the role of autophagy which acting as a seesaw between apoptosis and necroptosis increasing and decreasing changes in certain vital organs under effects of envenomation by Mesobuthus nigrocinctus and anti-venom administration in mice. In group design, we previously classified totally 42 Albino mice into two main categories including 1st, 3th and 6th hours of envenomation and anti-venom administrated animals (at dosages of 20LD50, 30LD50 and 40LD50) before standard envenomation procudure. These were sub-divided into six groups including envenomed and anti-venom administrated mice (n = 6 at each one) were studied as well as not being evenomed and not anti-venom-administrated mice (n = 6) were used as control group (n = 6). At the end of 24 h after envenomation, all animals were sacrificed. Liver, kidneys, spleen, heart and lungs tissues were collected from all groups. After routine histopathological examination, expressions of mTOR as an autophagy activator, expressions of RIPK3 as a necroptosis activator, and caspase-3, caspase-9 as the markers of apoptotic cell death signals were evaluated by immunoperoxidase method in addition to DNA in-situ fragmentations by TUNEL method in aforementioned tissues. In envenomed groups, caspases and TUNEL reactions were low after envenomation in contrast to high RIPK3 expressions. mTOR expressions were remained at stable. In anti-venom administration groups, mTOR and RIPK3 expressions were more decreased in contrast to higher caspases exspressions and TUNEL expressions. In conclusion, we think that the scorpion envenomation drift solely the cells to necroptosis. And, under presence of anti-venom supply, the decreased mTOR expressions triggered autophagy and dependently that apoptosis was developed instead of necroptosis. We believed that this venom-antivenom administration study is a usefull role-model for better understanding of switch effect of autophagy between apoptosis and necroptosis.
1. Introduction
Cell death mechanisms are still controversial for a long time whether or not this is a real self suicide or only a biological result or reaction against lethal factors. Therefore, there have been produced several terminologies meeting to those mechanism [1,2,3]. Autophagy machinery or self-eating mechanism depending to exposure duration or amount of disturbing cellular balance such as toxic agents can be switched or be resulted in apoptosis and necroptosis mechanism in the cell [3]. Oxidative stress such as Reactive Oxidative Substance (ROS) activates particullarly mitochondrial damages as well as other organells and molecule-like proteins. Scorpion venoms are one of them causing to oxidative stress on cells and mitochondrial instability depending on overproduction of ROS. Against the situation, autophagy machinery is activated [4,5]. mTOR (mammalian target of rapamycin), a serine/threonine kinase, is likely to a chief of orchestery. mTOR inhibition creates in autophagy induction [6]. If the damages are uncontrolled, mitochondrial distress is triggered. The mechanism, in this case, continued by serine–threonine kinase receptor-interacting protein (RIP) [7,8,9]. In the fact that caspases, furthermore, have a role of cleaving in cellular proteins to be like in RIPK1-RIPK3 complex in necrosome of dying cells [10,11]. Therefore, caspases take a role in both apoptotic and non-apoptotic molecular interactions [11].
Herein, we tried to show autophagy-apoptosis and necroptosis relations in various vital organs utilizing from envenomation modelling by Mesobuthus nigrocinctus and anti-venom administration at different dosages in mice.
2. Materials and Methods
Mesobuthus nigrocinctus were collected and its venom was milked as previously described by Ozkan et al., 2007 [12]. The protein content of the supernatant was assayed by Bradford method (1976) using BSA as standard. It was found to be 2.87 mg/mL by this method and 0.37 mg/kg by the Probit analysis. And, LD50 of venom was found to be 0.38 mg/kg. ED50 was found to be 41LD50/mL anti-venom. In modelling, 42 healthy CD1 Albino mice (18–22 g) were selected for two main categorizes including envenomed mice and anti-venom administrated mice before the envenomation. 18 mice were sacrificed in 1st, 3rd and 6th hours after envenomation (n = 6 for each time). In 18 mice, anti-venom procedure administrated as 20LD50, 30LD50 and 40LD50 doses. In control group, mice (n = 6) were not treated. Liver, kidneys, spleen, heart and lungs were collected and fixed in 10% neutral formalin. The sections were stained according to Haematoxylin-Eosin staining. For detection of mTOR, RIPK3, caspase-3 and caspase-9 expressions, strep Avidin-Biotin Complex Peroxidase (strep ABC-P) method was used (Peroxidase Detection System, RE7110-K, Leica, Wetzlar, Germany, Novocastra). Thereafter, TUNEL staining (terminal deoxynucleotidyl transferase mediated nick end labeling) assay method was applied according to the kit procedure (In situ Cell Detection Kit, Roche, cat no: 11684795910, Indianapolis, IN, USA,). All results were scored statistically.
3. Results
3.1. Histopathological Findings
In GII and GIII of envenomed subgroups, the degenerative and necrotic changes were increased when compared in that of GI (Figure 1a–c,h–j,o–r). Anti-venom administrated subgroups in envenomed mice, GII and GIII findings were more decreased (Figure 1 d,f,k–m,s–u). 3.1.3. Control group was normal (Figure 1g,n,v).
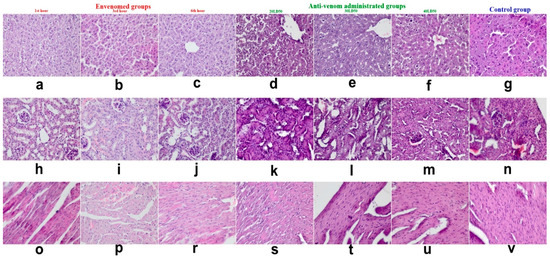
Figure 1.
Description of degeneration in liver in envenomed groups (a–c) and anti-venom groups (d–f), control group (g); kidneys in envenomed groups (h–j) and antivenom groups (k–m), control group (n); heart in envenomed groups (o–r) and antivenom groups (s–u), control group (v), x400, Hematoxylin-Eosin (H&E) staining.
3.2. Immunohistochemical Findings
mTOR expression, in envenomed subgroup, the expressions were not common. In anti-venom administrated subgroups, the expressions were increased in GI to GIII. There was meaningful statistical difference in both groups (p < 0.05). Caspase-3 and caspase-9 expressions, in envenomed subgroups, expressions were decreased. In anti-venom administrated subgroups, the expressions were more elevated. TUNEL reactions were similar to caspase expressions. But, RIPK3 expressions in envenomed subgroups were increased. In anti-venom administrated subgroups, the expressions were more decreased (p < 0.05). In control group, there were no expressions (p < 0.05 between this and other groups). All those expressions were given in the illustration. (Figure 2).
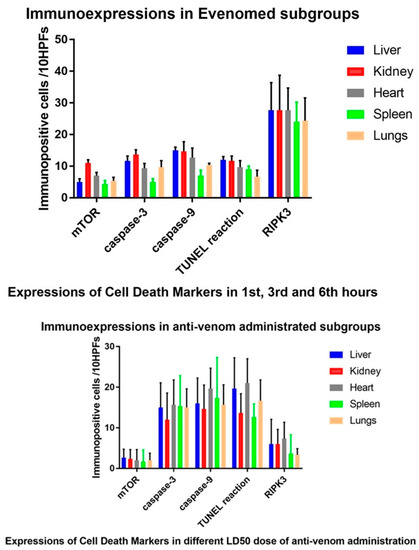
Figure 2.
Immunoexpressions of mTOR, caspases, DNA in situ fragmentation and RIPK3 in experimental groups according to mean calculation of time elapsing and anti-venom doses.
4. Discussion and Conclusions
In the current study, we come to some results that (i) there is a close relationship between autophagy-apoptosis and necroptosis. (ii) Mesobuthus nigrocinctus venom and anti-venom administration into mice is a usefull model for better understanding potential harmfull effect on cell in various vital organs. (iii) High LD50-doses of anti-venom might reverse the potential necroptotic effects on cells due to envenomation even though time-course of envenomation is passing out. (iv) caspase signals apart from apoptotic cell death can also aid in decreasing mTOR expressions in envenomed animals. So, combined expressions may trigger the activation of autophagy in higher anti-venom treatment. (v) RIPK3 may solely change the fate of cells in the course of necroptosis. In conclusion, we believe that the obtained results are the vast challenge to be understood of relations amongst different cell death mechanisms.
Author Contributions
M.E.A. and Ö.Ö. conceived and designed the experiments. M.E.A. performed analysis and preparing of the paper. Ö.Ö. performed the experiments.
Acknowledgments
There is no any funding resource or grants for the study. The study has been performed by authors’ own facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Walsh, C.M. Grand challenge since death and survival: Apoptosis vs. necroptosis. Front. Cell Dev. Biol. 2014, 2, 1–4. [Google Scholar]
- Lalaoui, N.; Lindqvist, L.M.; Sandow, J.J.; Ekert, P.G. The Molecular Relationships between Apoptosis, Autophagy and Necroptosis. Semin. Cell Dev. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Goodall, M.G.; Filtwalter, B.; Zahedi, S.; Wu, M.; Rodriguez, D.; Mulcahy-Levy, J.M.; Green, D.R.; Morgan, M.; Cramer, S.D.; Thorburn, A. The Autophagy Machinery Controls Cell Death Switching between Apoptosis and Necroptosis. Dev. Cell. 2016, 37, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Béchohra, L.; Laraba-Djebari, F.; Hammoudi-Triki, D. Cytotoxic activity of Androctonus australis hector Venom and Its Toxic Fractions on Human Lung Cancer Cell Line. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Naserzadeh, P.; Mehr, S.N.; Sadabadi, Z.; Seydi, E.; Salimi, A.; Pourahmad, J. Curcumin Protects Mitochondria and Cardiomyocytes from Oxidative Damage and Apoptosis Induced by Hemiscorpius Lepturus Venom. Drug Res. 2018, 68, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Colombi, M.; Moroni, C.; Hall, M.N. Rapamycin Passes the Torch: A New Generation of mTOR Inhibitors. Nat. Rev. Drug Discov. 2011, 10, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Han, W.; Li, L. Targeting the weak point of cancer by induction of necroptosis. Autophagy 2007, 3, 490–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horita, H.; Frankel, A.E.; Thorburn, A. Acute Myeloid Leukemia-Targeted Toxin Activates Both Apoptotic and Necroptotic Death Mechanisms. PLoS ONE 2008, 3, e3909. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X. Mixed Lineage Kinase Domain-Like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.A.; Clarke, P.R. Apoptosis and Autophagy: Regulation of Caspase-9 by Phosphorylation. FEBS J. 2009, 276, 6063–6073. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular Mechanism Controlling Caspase Activation and Function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, O.; Ciftci, G.; Pekmezci, G.Z.; Kar, S.; Uysal, H.; Karaer, K.Z. Proteins, lethality and in vivo effects of Iurus dufoureius asiaticus scorpion venom. Toxicon 2007, 50, 394–399. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).