Lignocellulosic Biomass as a Source of Raw Materials for the Synthesis of Polyurethanes †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S. Catalytic conversion of lignocellulosic biomass to fine chemical and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Correa, T.; Martin, M.D.; Retegi, A.; Gabilondo, N.; Corcuera, M.A.; Eceiza, A. Synthesis and characterization of polyurethane with high renewable carbon content and tailored properties. ACS Sustain. Chem. Eng. 2016, 4, 5684–5692. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Roncal, T. Production of sorbitol from Biomass. In Production of Platform Chemicals from Renewable Resources; Biofuels and Biorefineries; Springer: Singapore, 2017; Volume 7, Chapter 9. [Google Scholar]
- Roncal-Martínez, T.; Caballero, T.; Díaz de Guereñu-Zabarte, M.M.; Rincón-Arroyo, I.; Prieto-Martínez, S.; Ochoa-Gómez, J.R. Bacterial strain producing 2,3-butanediol and other metabolites. Eur. Pat. Appl. EP16382347 2016. [Google Scholar]
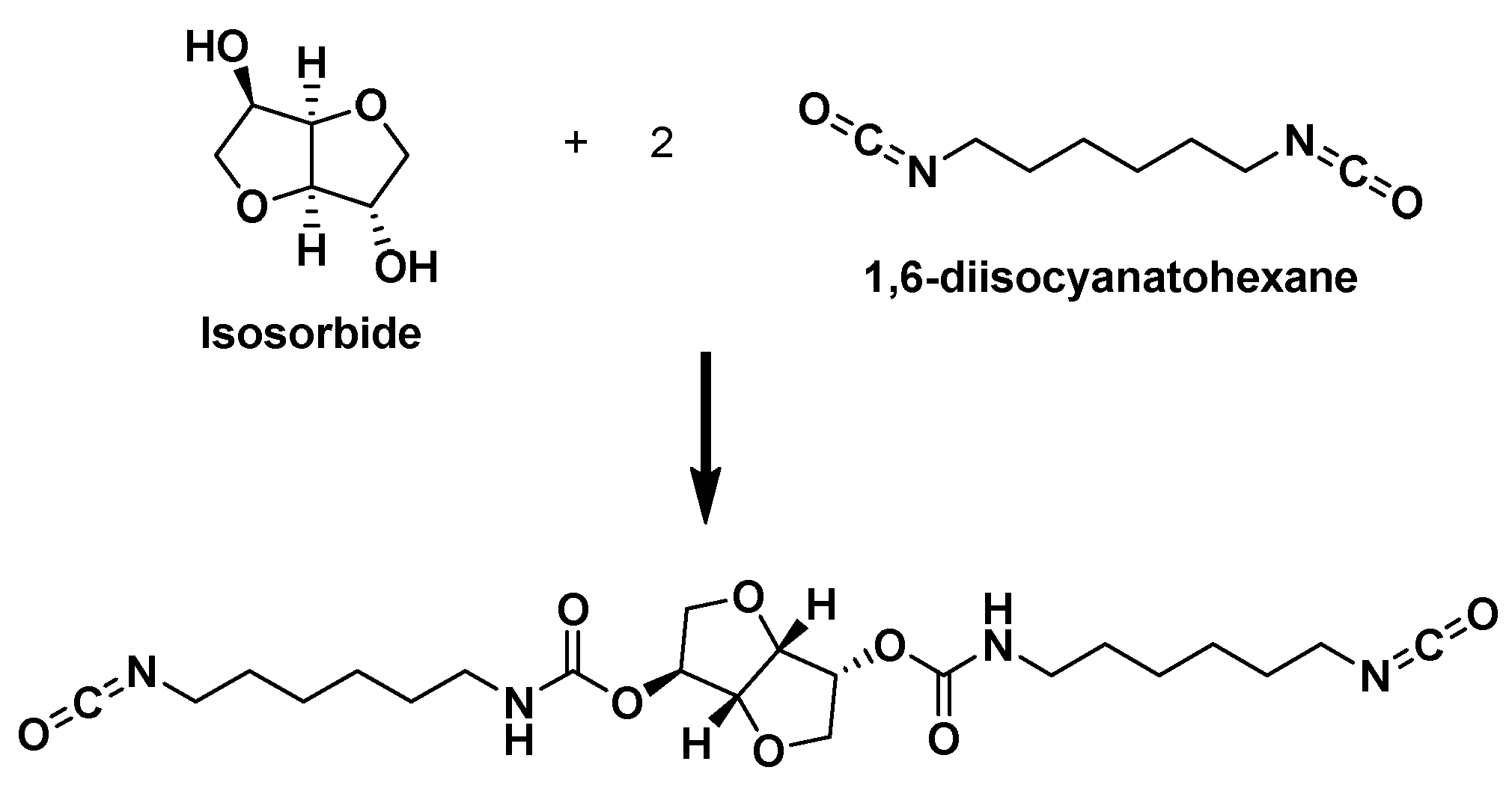
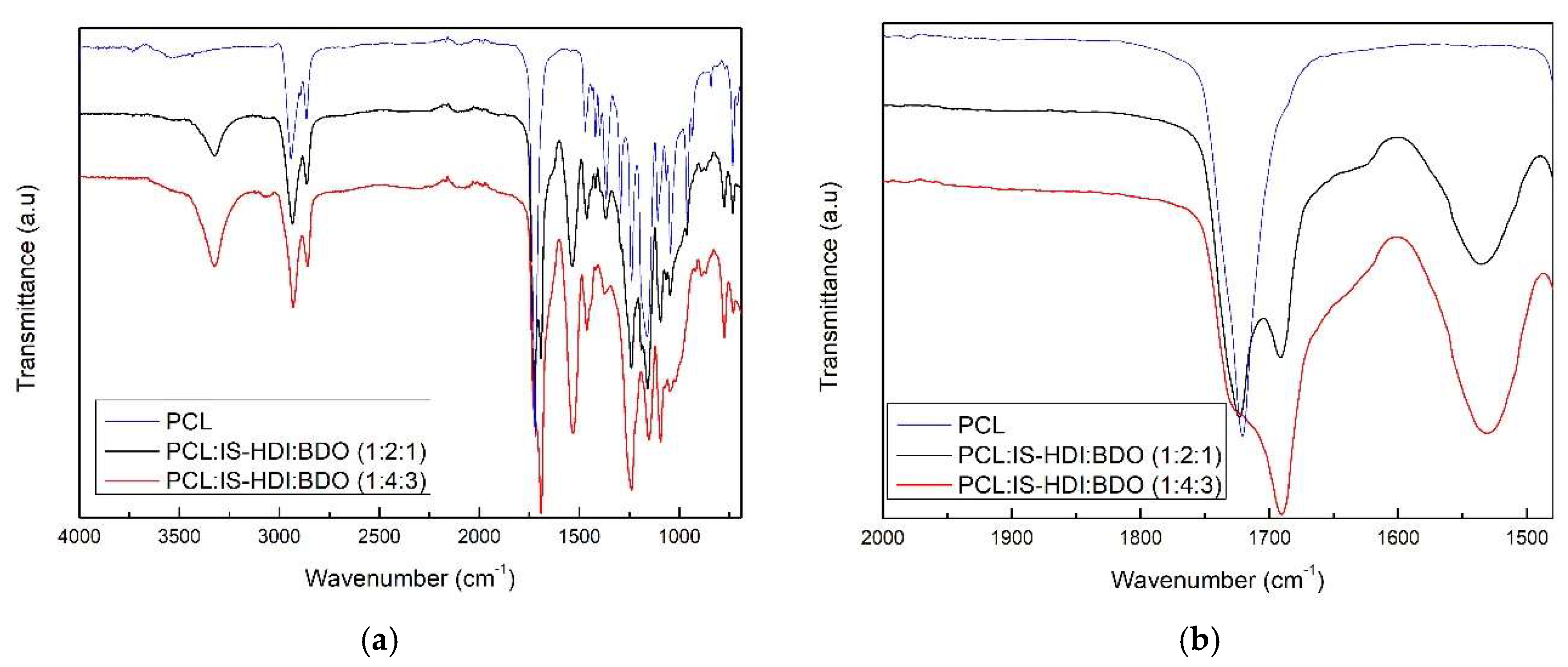

| Samples | PCL:IS-HDI:2,3-BDO Molar Ratio | IS-HDI (wt.%) | HS (wt.%) |
|---|---|---|---|
| PCL:IS-HDI:2,3-BDO | 1:2:1 | 41.05 | 43.65 |
| PCL:IS-HDI:2,3-BDO | 1:4:3 | 56.15 | 61.48 |
| Samples | Tensile Modulus (MPa) | Stress at Break (MPa) | Strain at Break (%) |
|---|---|---|---|
| PCL: IS-HDI: 2,3-BDO (1:2:1) | 170.9 ± 23.4 | 7.9 ± 1.3 | 15.1 ± 5.1 |
| PCL: IS-HDI: 2,3-BDO (1:4:3) | 355.1 ± 28.3 | 11.4 ± 1.8 | 264.8 ± 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Correas, T.; Ugarte, L.; Ochoa-Gómez, J.R.; Roncal, T.; Diñeiro, C.; Corcuera, M.A.; Eceiza, A. Lignocellulosic Biomass as a Source of Raw Materials for the Synthesis of Polyurethanes. Proceedings 2018, 2, 1493. https://doi.org/10.3390/proceedings2231493
Calvo-Correas T, Ugarte L, Ochoa-Gómez JR, Roncal T, Diñeiro C, Corcuera MA, Eceiza A. Lignocellulosic Biomass as a Source of Raw Materials for the Synthesis of Polyurethanes. Proceedings. 2018; 2(23):1493. https://doi.org/10.3390/proceedings2231493
Chicago/Turabian StyleCalvo-Correas, Tamara, Lorena Ugarte, José R. Ochoa-Gómez, Tomás Roncal, Cristina Diñeiro, Maria Angeles Corcuera, and Arantxa Eceiza. 2018. "Lignocellulosic Biomass as a Source of Raw Materials for the Synthesis of Polyurethanes" Proceedings 2, no. 23: 1493. https://doi.org/10.3390/proceedings2231493
APA StyleCalvo-Correas, T., Ugarte, L., Ochoa-Gómez, J. R., Roncal, T., Diñeiro, C., Corcuera, M. A., & Eceiza, A. (2018). Lignocellulosic Biomass as a Source of Raw Materials for the Synthesis of Polyurethanes. Proceedings, 2(23), 1493. https://doi.org/10.3390/proceedings2231493






