Abstract
The use of hydrogen as an energy vector seems today one of the most viable alternatives, although its use involves safety problems due to the generation of explosive atmospheres. Lower and upper flammability limits are one of the most important parameters at the time of characterize and handle flammable gases. This paper is focused on the evaluation of the safety prevention measures more commonly applied to explosive atmospheres, specifically the inertization, by means of a non-flammable gas (CO2) in air-hydrogen mixtures. In this study both theoretical and experimental methods to determine the flammability interval have been carried out. The flammability limits and the LOC value are represented in a ternary diagram enable to determine the explosive area containing the entire range of explosive mixtures. The experimental study has been conducted in a flameproof chamber of 0.5 dm3 volume, designed to withstand explosions inside. The results show that the theoretical approaches are only valid as a previous step to the experimental tests, since the observed differences between both implies that the use of such approaches could lead to important safety risks.
1. Introduction
The great increase in energy demand occurred in recent decades has led to an excessive increase in the consumption of fossil fuels, which irremediably has a large impact on the environment.
This scenario leads to the need to raise new energy systems that are capable to palliate these problems. The challenge to the use of hydrogen as an energy vector seems today to be one of the most viable alternatives [1], although its use still poses major safety problems, due to the formation of explosive atmospheres, which results indispensable to study.
The solution to these problems departs from the study of the flammability characteristics of hydrogen, being the most important and significant the Flammability Limits (FL), lower (LFL) and upper (UFL). These are characteristics measured in binary fuel-combustion mixtures. If an inert gas is added to such binary mixture, it displaces oxygen volume, depending on the concentration, the mixture could be completely inerted. This method is commonly used in industry, being defined by the limiting oxygen concentration (LOC). This will be the threshold value from which the atmosphere is totally inert, thus preventing explosion propagation.
By means of the graphic representation of the FL in triangular diagrams, it is possible to determine the explosive region of the mixture, which will be located within the triangular area delimited by the UFL, LFL and LOC.
The values of flammability limits for a hydrogen-air mixture that have been collected to carry out this study are 4% LFL and 75% UFL [2]. Based on these data, there are numerous studies for the LOC value to determine threshold concentration and thus being able to take safety measures to work with these dangerous mixtures [3].
If, on the other hand, a point curve is obtained that contains the set of explosive ternary mixtures, it is possible to adjust with greater precision and criterion the safety elements, which would lead to reducing costs in the application of safety measures and would work in a completely safety way.
The study intends to obtain therefore such a curve for the mixture hydrogen-air-carbon dioxide, first by means of a theoretical approach and after by a modest experimental method. The choice of this inert is based on the fact that CO2 is more effective against hydrogen than other mostly used gases, like nitrogen. This is due to the inerting power increases as the calorific value (Cp) of the gas increases, being CO2 calorific value greater than N2.
2. Materials and Methods Section
Theoretically, it is possible to carry out an empirical approximation of the flammability interval, by approximate LOC calculation.
Flammability interval of a fuel-combustion-inert gas mixture is located, in the triangular diagrams, between the explosibility limits and the corresponding vertex of the inert gas. If they linked by straight lines, a triangular region is obtained that will separate the explosive region (inside the triangular area) and the non-explosive region (outside the triangular area). This differentiation between both regions is very imprecise, since the explosive interval closes in the form of a parabola before reaching the axis of the inert gas [4], drawing a curve encloses the set of explosive mixtures. The vertex of such parabola corresponds to LOC. Theoretical determination of the LOC will therefore be the key issue to represent the desired flammability interval.
Lewis, B. and Von Elbe G [5] estimated that the LOC for a gaseous mixture of hydrogen, air, and carbon dioxide is 7.7% of oxygen concentration. It would then be possible to inert a mixture of hydrogen-air by adding 57% of CO2.
Besides these bibliographic data, it has been considered interesting to perform a graphical calculation from which it is possible to obtain an empirical approach.
For this, the flammability limits previously presented in the ternary diagram will be represented.
To obtain the LOC point, the stoichiometric point must be calculated first. To simplify calculations, the representation of the flammability interval will be carried out in a ternary system referred to pure oxygen (see Figure 1a). This will correspond to the stoichiometric concentration of fuel-combustion mixtures for which the combustion reaction is complete. For the studied system, the stoichiometric molar fraction of oxygen is 33% of concentration.
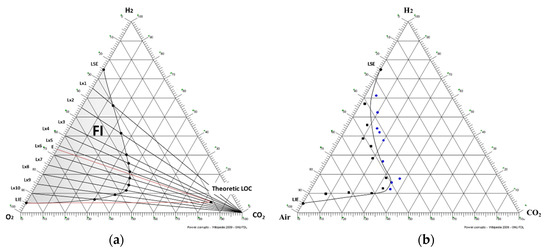
Figure 1.
(a) Theoretic Flammability Interval (FI), and (b) Experimental Flammability Interval.
From the previous explained point, the stoichiometric line will be drawn to the inert gas vertex, defining all those mixtures that, for a pure inert gas, the oxygen-fuel ratio is always stoichiometric.
Limiting oxygen concentration is located just at the intersection between stoichiometric line and LFLline. To obtain this second line, it will come from the hypothesis that adding an inert gas does not imply a variation in the LFL. The percentage independence of an added inert gas is reaffirmed if it is observed that LFL concentration in an air-hydrogen system is the same as in an oxygen-hydrogen system, remaining in both cases at 4% concentration. So, it can be assumed that LOC will be at the same height as LFL in a pure hydrogen and air oxygen system.
Therefore, as it has been explained, LOC is located on the intersection of both lines. This value, obtained in a reference system with pure oxygen, must be recalculated, through the concentration of oxygen in air, to the system studied in this document.
Once the theoretical Flammability Interval, FI has been obtained, as shown in Figure 1a, experimental tests will be carried out. Experimental development has been conducted in a flameproof chamber, designed to contain explosions inside. Volume of the gas chamber is 0.5 dm3. Inlet pressure of gases has been set at 2 bar. The ignition source used is a standard spark plug commonly used in internal combustion engines so that its energy is considered sufficient to carry out the ignition of the different mixtures tested. Explosion will be registered by a temperature sensor which measure temperature increase produced after the ignition. The criterion used to determine if explosion has occurred, is to register a temperature increase higher than 2 K. Experimental assembly described above has been carried out in a flameproof test tank used for tests according to the standard EN 60079 [6].
From theoretical curve, 10 lines are distributed approximately as shown in Figure 1a. The methodology carried out will consist in selecting different samples, on the defined lines, including the explosive area and the non-explosive area. By testing these mixtures, it will be possible to find the inflection point between both regions, being able to find the exact point belonging to the FI curve. This method is a variation of the one proposed in the standard EN 1839: 2017 (E) [7].
3. Results
Gases concentration will be one of the most important variables to control, so a calibration of the mixtures will be carried out by means of a paramagnetic oxygen analyzer. This mass controllers calibration determined that, for its correct operation, it was necessary to work with valve openings over 30%. Since the flameproof tank is designed to work with large volumes of gases, in many of the tested mixtures, work was carried out below this threshold value, then a deviation of up to 2.72% was observed between desired concentration to be tested and real concentration in the gas chamber. For this, tests were performed together with the oxygen analyzer, which allowed to carry out a reading of the actual concentration in the gas chamber. This supposes that the method to obtain the curve is less precise. The curve will split the flammable mixture and the non-flammable mixtures.
Theoretical curve was shown in Figure 1a, where the explosive area has named FI (Flammability Interval). Experimental results are also shown graphically in Figure 1b.
The Figure 1b shows only borderline tests, which delimit the curve of the flammability range. Each of these tests was repeated three times.
3.1. FI Comparision
The first thing that draws attention after experimental tests is that, having used an ignition source with an energy higher than the Minimum Ignition Energy (MIE) of hydrogen, it was expected to obtain a major flammability interval than the theoretical one. There are many factors which can affect explosive interval, knowing that both the pressure and the temperature and even the ignition energy imply an increase of the flammability interval [8,9]. Visually it has been observed that CO2, in addition to having a great inerting power, considerably reduces the severity of the explosion, ticking the flame and therefore the heat of reaction. Since explosion was monitored with a temperature sensor and with a very low explosion acceptance criterion, it can be assumed that some of the explosions closest to the real curve have not been detected and this will be the main reason of the result deviation.
An inverted parabola that was not foreseen in the theoretical curve is also observed. This means that the lower air concentration in mixture, the higher inerting power of CO2.
3.2. LOC Comparision
An experimental LOC of 11.45% of oxygen concentration has been obtained, while the collected bibliographic data placed it at around 7–8%. The lack of sensitivity of the sensor could be the cause of the difference between the two.
4. Conclusions
Firstly, it is concluded that CO2, in addition to inerting the mixture in terms of available oxygen reduction, acts also as a thermal sink [9] decreasing the temperature of the explosion, so the choice of the sensor will be a key aspect when determining the explosive region.
In addition, it has been seen that it is possible to carry out theoretical approximations that will give an approximate idea of the safe region when working with flammable gases. Applying a very high safety factor would make it possible to even work with these approaches in practice. Even so, analyzing the results it can be concluded that it is most appropriate to carry out experimental tests that determine exactly the explosive region, since correctly adjusting the explosive interval entails the reduction of costs when applying preventive measures, and this means to work in safe conditions.
Acknowledgments
To the Laboratorio Oficial Madariaga for allowing me to use its facilities and laboratories and all its personnel for always helping me.
References
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Cashdollar, K.L.; Zlochower, I.A.; Green, G.M.; Thomas, R.A.; Hertzberg, M. Flammability of methane, propane, and hydrogen gases. J. Loss Prev. Process Ind. 2000, 13, 327–340. [Google Scholar] [CrossRef]
- Zlochower, I.A.; Green, G.M. The limiting oxygen concentration and flammability limits of gases and gas mixtures. J. Loss Prev. Process Ind. 2009, 22, 499–505. [Google Scholar] [CrossRef]
- Lewis, B.; Von Elbe, G. Combustion, Flames and Explosions of Gases, 2nd ed.; Elsevier: London, UK, 1961. [Google Scholar]
- UNE-EN.70069-1; Explosive Atmospheres—Part 1: Equipment Protection by Flameproof Enclosures “D”. International Electrotechnical Commission: Geneva, Switzerland, 2014.
- EN 1839:2017 (E); Determination of the Explosion Limits and the Limiting Oxygen Concentration (LOC) for Flammable Gases and Vapours. International Electrotechnical Commission: Geneva, Switzerland, 2017.
- Liu, X.; Zhang, Q. Influence of initial pressure and temperature on flammability limits of hydrogen-air. Int. J. Hydrog. Energy 2014, 39, 6774–6782. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Wang, X.; Lin, Y. Experimental and theoretical study on the influence of temperature and humidity on the flammability limits of ethylene (R1150). Energy 2013, 52, 185–191. [Google Scholar] [CrossRef]
- García Torrent, J. Seguridad Industrial En Atmósferas Explosivas; Escuela Técnica Superior de Ingenieros de Minas: Madrid, Spain, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).