Study of Siderurgy Slags for the Reuse of Iron Oxides for Biotechnology Application †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chand, S.; Paul, B.; Kumar, M. Sustainable Approaches for LD Slag Waste Management in Steel Industries: A Review. Metallurgist 2016, 60, 116–128. Available online: https://link-springer-com/article/10.1007/s11015-016-0261-3 (accessed on 26 May 2018). [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An Overview of Utilization of Slag and Sludge from Steel Industries. Resour. Conserv. Recycl. 2007, 50, 40–57. Available online: https://www.sciencedirect.com/science/article/pii/S0921344906001297 (accessed on 26 May 2018). [CrossRef]
- Branca, T.; Pistocchi, C.; Colla, V.; Ragaglini, G.; Amato, A.; Tozzini, C.; Mudersbach, D.; Morillon, A.; Rex, M.; Romaniello, L. Investigation of (BOF) Converter Slag Use for Agriculture in Europe. Metal. Res. Technol. 2014, 111, 155–167. Available online: https://www.metallurgical-research.org/articles/metal/abs/2014/03/metal140009/metal140009.html (accessed on 26 May 2018). [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nano Res. Lett. 2008, 3, 397–415. Available online: https://nanoscalereslett.springeropen.com/articles/10.1007/s11671-008-9174-9 (accessed on 26 May 2018). [CrossRef] [PubMed]
- Sun, S.H.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. Available online: http://science.sciencemag.org/content/287/5460/1989.long (accessed on 26 May 2018). [CrossRef] [PubMed]
- Jena, B.C.; Dresler, W.; Reilly, I.G. Extraction of Titanium, Vanadium and Iron from Titanomagnetite Deposits at Pipestone Lake, Manitoba, Canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Aaron, M.J.; Heather, C.A. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. Appl. Mater. Interfaces 2010, 2, 2804–2812. Available online: https://pubs.acs.org/doi/abs/10.1021/am1004943 (accessed on 26 May 2018). [CrossRef]
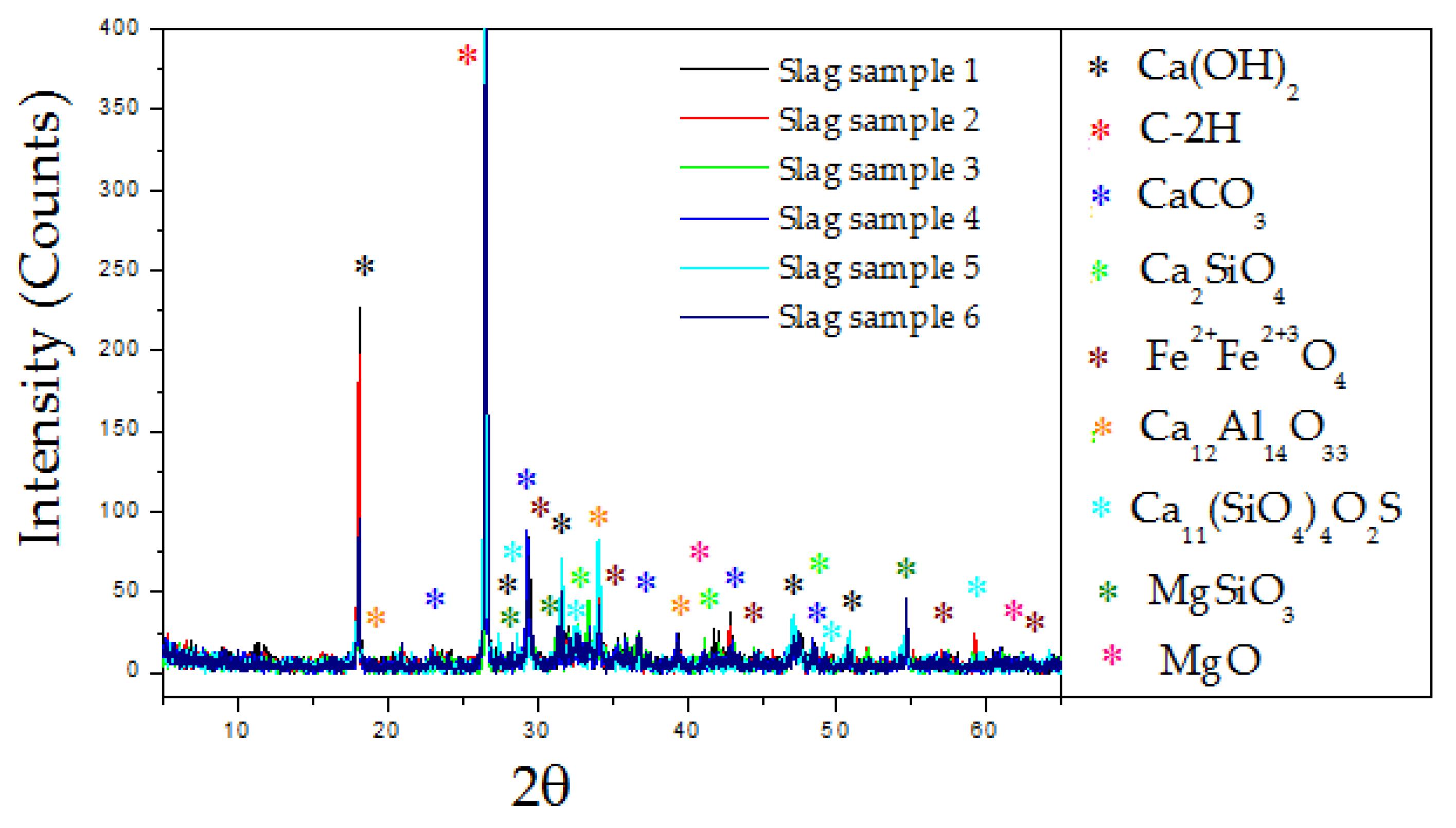

| Composition | Slag Sample 1 | Slag Sample 2 | Slag Sample 3 |
|---|---|---|---|
| Ca(OH)2 | 12.3% | 15.4% | 9.5% |
| 2H–C | 10.1% | 15.5% | 26.1% |
| CaCO3 | 55.6% | 54.2% | 42.5% |
| Ca2SiO4 | 8% | 4.1% | 13.3% |
| Fe3O4 | 2.3% | 3.3% | 1.7% |
| MgO | 8.3% | 2.8% | - |
| Ca12Al14O33 | 3.3% | 4.7% | - |
| MgSiO3 | - | - | 6.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, D.T.; Oliveira, A.H.; Batista, A.S.M. Study of Siderurgy Slags for the Reuse of Iron Oxides for Biotechnology Application. Proceedings 2018, 2, 1304. https://doi.org/10.3390/proceedings2201304
Moreira DT, Oliveira AH, Batista ASM. Study of Siderurgy Slags for the Reuse of Iron Oxides for Biotechnology Application. Proceedings. 2018; 2(20):1304. https://doi.org/10.3390/proceedings2201304
Chicago/Turabian StyleMoreira, Diogo T., Arno H. Oliveira, and Adriana S. M. Batista. 2018. "Study of Siderurgy Slags for the Reuse of Iron Oxides for Biotechnology Application" Proceedings 2, no. 20: 1304. https://doi.org/10.3390/proceedings2201304
APA StyleMoreira, D. T., Oliveira, A. H., & Batista, A. S. M. (2018). Study of Siderurgy Slags for the Reuse of Iron Oxides for Biotechnology Application. Proceedings, 2(20), 1304. https://doi.org/10.3390/proceedings2201304





