Abstract
Present day steelmaking slags are being successfully used as a high quality mineral aggregate for the building industry. With this, it is of vital importance to be familiar with the technical significance of the secondary application of steel slag, because some steel slag might contain increased concentration of substances harmful to human health. In terms of steel slag impact on the environment, radionuclides are the least researched of all pollutants emitted from the metallurgical processes. This work presents the preliminary study about the presence of the uranium in siderurgy slag aggregates for the purpose of its use in the production of construction material. The results showed that this slag is free of uranium which brings greater security in its use as building material.
1. Introduction
Many areas in the world have elevated naturally occurring radioactive materials (NORM) caused either by the geological and geochemical structure of the soil, or by the radioactive content of the water flowing from hot springs and/or due to technologically enhanced radioactivity as well as due to cosmic rays [1]. Human manipulation of NORM for economic ends may lead to what is known as “technologically enhanced naturally occurring radioactive materials”, often called TENORM. The existence of TENORM results in an increased risk for human exposure to radioactivity. TENORM industries may release significant amounts of radioactive material into the environment resulting in the potential for widespread exposure to ionizing radiation. These industries include mining, phosphate processing, metal ore processing, heavy mineral sand processing, titanium pigment production, fossil fuel extraction and combustion, manufacture of building materials, thorium compounds, aviation, and scrap metal processing [2].
The Ferriferous Quadrilateral encompasses an area of ca. 7000 km2 in the central part of the state of Minas Gerais (southeastern Brazil) and it is rich in mineral occurrences being considered one of the richest mineral-bearing regions in the world [3,4]. A previous study related that this region it has an abundance of iron oxides in the clay fraction decreasing in the following sequence: hematite, goethite, maghemite. Studies about of uranium contents in hematite concentrates confirm the presence of the uranium as trace-element [5]. In the context can indicate presence of the uranium’s sons, as radium and radon. The anthropogenic activities, as the mining industry, can elevate the exposition environment this natural radioactivity.
Slags from different metallurgical processes contain many useful components used for various industrial and construction purposes. The properties of slag including mineralogical composition in conform to steel-making operations. Basic Oxygen Furnace Process (BOF), Electric Arc Furnace (EAF) and Open Hearth Furnace (OHR) produce slags of different compositions. A study the radiochemical characteristics of EAF slag from Croatian EAF black steel slags demonstrated presence of natural isotopes 40K, 232Th, 228Ra, 226Ra and 238U, although the measured activity in slag natural isotopes lies within the Croatian legally permitted limits [6]. Another study in measurement of the natural radionuclide content in several samples of Romanian metallurgical slag, detected gamma-ray emitting radionuclides, which belong to the natural radioactive series: 238U, 232Th, 235U and 40K [7]. Therefore, for the safe reuse of steel slag it is necessary to carefully investigate the possible presence of radionuclides in order to prevent damages as to increased exposure to natural radioactivity.
2. Materials and Methods
The test has been conducted on steel slag created during the product of Oxygen Furnace Process (BOF) by Linz-Donawitz (LD) process in the siderurgy localized in Ferriferous Quadrilateral of Minas Gerais, southeastern Brazil. Five samples were collected in the steel mill slag yards as a remnant of the transformation of pig iron into steel. The material was prepared for granulometric reduction with 0.210 mm sieve opening using a sieve Granutest® and a sixth sample was prepared by mixing the others. Scanning Electron Microscope (SEM) and Energy Dispersive X-ray Spectrometer (EDS) was performed at a SIGMA VP field emission scanning electron microscope ZEISS. For XRD diffractogram of the samples, we used the diffractmeter Rigaku, model and system D\MAX ULTIMA automatic, with theta-theta goniometer (θ-θ) and 2θ/θ scan with 2θ detector and copper anode, with X-ray energy beams of 8.04 keV. Samples were being homogenously dispersed with KBr powder for prepared of the disks for Fourier Transform Infrared Spectroscopy (FTIR). FTIR analyses was performed with a BOMEM spectrometer, each collected with 32 scans each, for wavenumbers ranging from 300 to 4000 cm−1.
3. Results and Discussion
For proper reuse of the siderurgy slag as aggregate in cements, for example, it is necessary to distinguish the mineral forms present to determine its volumetric instability and therefore it is common to use the EDS and X-ray diffraction analysis for its proper characterization. EDS data by SEM images specifies the chemical composition of the aggregate and show expressive constitution in oxygen and calcium. This is expected because the LD process uses gaseous oxygen to refine the liquid pig iron, reducing the carbon, silicon, manganese, phosphorus and sulfur, through the oxidation of these elements.
The XRD technique can also be used to investigate the presence of radioactive minerals, such as uranium or thorium, although studies of this nature are less frequent. In EDS data we observed the elemental composition of the slag studied and a comparison with Figure 1 helps to understand how these elements are related in the formation of mineral phases. Figure 1 shows the diffractogram of one of the samples collected in the slag stack with the correlation of the peaks presented with characteristic peaks of known mineral phases. It is shown only a diffractogram for better visualization of the peaks and for being similar to the others.
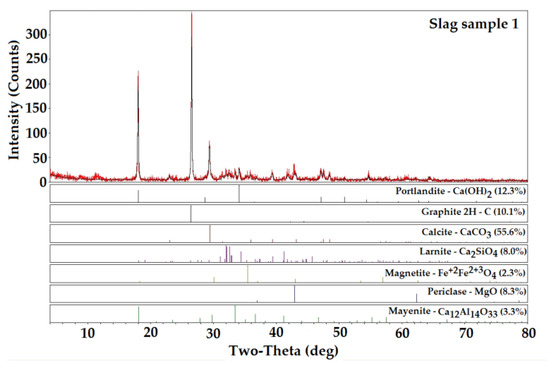
Figure 1.
XRD diffractogram of the slag sample with signaling of the characteristic peaks of the minerals identified.
FTIR analysis was used to study the absorption peaks characteristic of U3O8, UO3 and UO2. In the other study FTIR was used to identify U3O8 in samples of UO2 powder. UO2 can be identified by bands at 340 cm−1 and 470 cm−1, and U3O8 and UO3 by bands at 735 cm−1 and 910 cm−1, respectively [8]. In this work we observed in Figure 2a FTIR absorption bands between 300 cm−1 and 2000 cm−1 that are attributed to minerals signalized by the XRD technique, such as calcite, portlandite and larnite. Unfortunately, among these bands are the peaks investigated in the search for uranium ores. A more detailed view in Figure 2b, where dashed lines indicate the location of the peaks under study, which refer to uranium oxides, we note the absence of a prominent peak at 735 cm−1 and 910 cm −1. On the other hand absorbance peaks at 340 cm−1 and 470 cm−1 are located like a broad peak in absorption region characteristic of iron oxides, which makes it difficult to assign to uranium oxides.
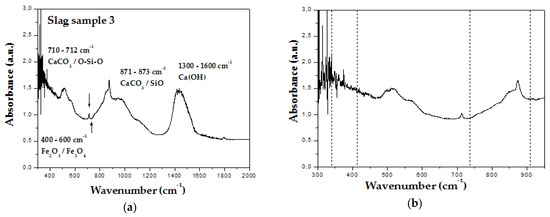
Figure 2.
FTIR spectra of the: (a) slag sample 3 for wavenumbers ranging from 300 to 2000 cm−1; (b) slag sample 1 for wavenumbers ranging from 300 to 750 cm−1.
4. Conclusions
The analyzes used in this study demonstrate that the siderurgy slag is free of uranium oxides; although more sensitive analyzes of the presence of traces can determine the presence of uranium in small proportions with better precision. On the other hand, for radiation safety purposes, small portions of uranium in large quantities of materials, such as those generated daily in steel production, may not really be associated with increased exposure to natural radionuclides. Other authors, who carried out similar studies, but with radiochemical techniques, that is, more specific for this type of investigation, were able to identify the presence of radionuclides, but all in proportions below the acceptable levels, compared to the levels already naturally present in the environment. Therefore, although the techniques chosen for this work are not specific for observation of radiation emitting materials, they could be the most appropriate choice, since a positive result regarding the presence of radionuclides would already signal the need for safety intervention.
Author Contributions
A.S.M.B. and A.H.O. conceived and designed the experiments; D.T.M. performed the experiments; A.S.M.B. analyzed the data; D.T.M. contributed reagents/materials/analysis tools; A.S.M.B. wrote the paper.
Acknowledgments
Financial support from Conselho Nacional de Pesquisa e Desenvolvimento Científico (CNPq) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) are acknowledged.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Mehdi, S. The state-of-the-art on worldwide studies in some environments with elevated naturally occurring radioactive materials (NORM). Appl. Radiat. Isotopes 1998, 49, 169–188. [Google Scholar] [CrossRef]
- Vearrier, D.; Curtis, J.A.; Greenberg, M.I. Technologically enhanced naturally occurring radioactive materials. Clin. Toxicol. 2009, 47, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.A.B.C.; Palmieri, H.E.L.; Leonel, L.V.; Nalini, H.A., Jr.; Jaćimović, R. Iron Quadrangle, Brazil: Elemental concentration determined by k0-instrumental neutron activation analysis Part I: Soil samples. J. Radioanal. Nucl. Chem. 2006, 270, 111–116. [Google Scholar] [CrossRef]
- Carvalho Filho, A.; Inda, A.V.; Fink, J.R.; Curi, N. Iron oxides in soils of different lithological origins in Ferriferous Quadrilateral (Minas Gerais, Brazil). Appl. Clay Sci. 2015, 118, 1–7. [Google Scholar] [CrossRef]
- Kessler, W.; Müller, G. Minor and trace-element data of iron oxides from iron-formations of the Iron Quadrangle, Minas Gerais, Brazil. Miner. Petrol. 1988, 39, 245–250. [Google Scholar] [CrossRef]
- Sofilić, T.; Barišić, D.; Sofilić, U. Natural radioactivity in steel slag aggregate. Arch. Metall. Mater. 2011, 56, 627–634. [Google Scholar] [CrossRef]
- Sahagia, M.; Luca, A.; Antohe, A.; Ioan, R.; Tanase, M.; Torano, E.G. Comparison of analysis methods for the characterisation of the radioactive content of metallurgical slag used within the EURAMET-EMRP JRP IND04 metrometal. Rom. Rep. Phys. 2014, 66, 649–657. Available online: http://www.rrp.infim.ro/2014_66_3/A6.pdf (accessed on 31 May 2018).
- Silva, L.A.; Lameiras, F.S.; Santos, A.M.M.D.; Ferraz, W.B.; Barbosa, J.B.S. Determination of U3O8 in UO2 by infrared spectroscopy. REM Int. Eng. J. 2017, 70, 59–62. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).