Microwave Heating Impact on the Oil Yield from Botryococcus braunii Algae Biomass †
Abstract
:1. Introduction
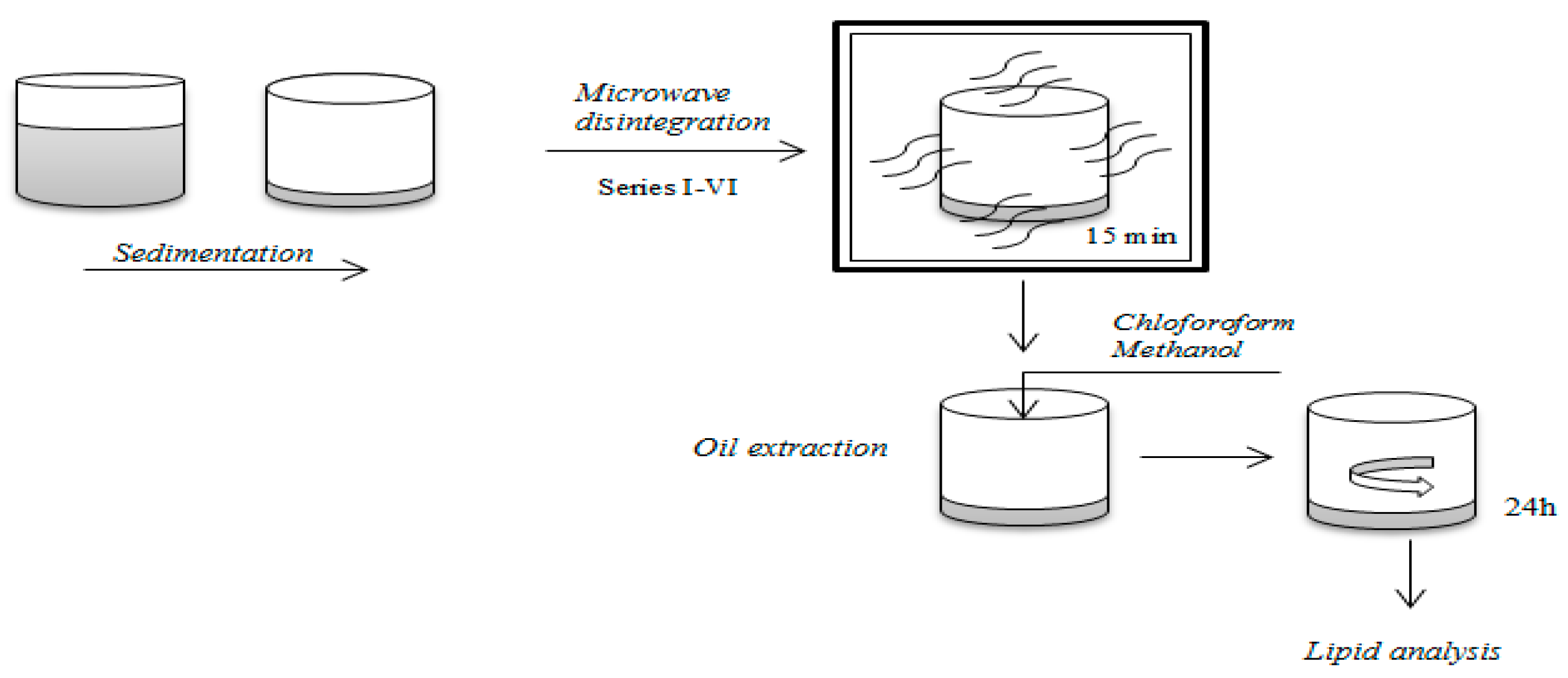
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tasić, M.B.; Pinto, L.F.R.; Klein, B.C.; Veljković, V.B.; Maciel Filho, R. Botryococcus braunii for biodiesel production. Renew. Sustain. Energy Rev. 2016, 64, 260–270. [Google Scholar] [CrossRef]
- Hirose, M.; Mukaida, F.; Okada, S.; Noguchi, T. Active hydrocarbon biosynthesis and accumulation in a green alga, Botryococcus braunii (Race A). Eukaryot. Cell 2013, 12, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Macías-Sánchez, M.D.; Robles-Medina, A.; Jiménez-Callejón, M.J.; Hita-Peña, E.; Estéban-Cerdán, L.; González-Moreno, P.A.; Navarro-López, E. Optimization of Biodiesel Production from Wet Microalgal Biomass by Direct Transesterification Using the Surface Response Methodology. Renew. Energy 2018. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.K. Bioresource Technology Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Microalgal fatty acids—From harvesting until extraction. Microalgae-Based Biofuels Bioprod. 2017, 369–400. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, C.; Jun, S.; Ahn, C.; Oh, H. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.K.; Yen Doan, T.T.; Obbard, J.P. Factors affecting cellular lipid extraction from marine microalgae. Chem. Eng. J. 2013, 215–216, 929–936. [Google Scholar] [CrossRef]
- Kim, T.H.; Suh, W.I.; Yoo, G.; Mishra, S.K.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Development of direct conversion method for microalgal biodiesel production using wet biomass of Nannochloropsis salina. Bioresour. Technol. 2015, 191, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Arora, N.; Pruthi, V.; Pruthi, P.A. A Novel Rapid Ultrasonication-Microwave Treatment for Total Lipid Extraction from Wet Oleaginous Yeast Biomass for Sustainable Biodiesel Production. Ultrason. Sonochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Watson, I.A. Microwave treatment of wet algal paste for enhanced solvent extraction of lipids for biodiesel production. Renew. Energy 2015, 76, 470–477. [Google Scholar] [CrossRef]

| Control Sample | Series I | Series II | Series III | Series IV | |
|---|---|---|---|---|---|
| Temperature of incubation [°C] | 22 | 30 | 45 | 60 | 75 |
| Incubation time [min] | - | 15 min | |||
| Obtained oil [g] | 0.36 | 0.58 | 0.76 | 0.46 | 0.49 |
| Quantity of organic dry biomass used for the extraction [g o.d.m.] | 2.02 | ||||
| Percentage of oil in the biomass [% o.d.m.] | 17.82 | 28.71 | 37.62 | 22.77 | 24.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokicka, M.; Zieliński, M.; Dębowski, M.; Głowacka, K. Microwave Heating Impact on the Oil Yield from Botryococcus braunii Algae Biomass. Proceedings 2018, 2, 1289. https://doi.org/10.3390/proceedings2201289
Rokicka M, Zieliński M, Dębowski M, Głowacka K. Microwave Heating Impact on the Oil Yield from Botryococcus braunii Algae Biomass. Proceedings. 2018; 2(20):1289. https://doi.org/10.3390/proceedings2201289
Chicago/Turabian StyleRokicka, Magdalena, Marcin Zieliński, Marcin Dębowski, and Katarzyna Głowacka. 2018. "Microwave Heating Impact on the Oil Yield from Botryococcus braunii Algae Biomass" Proceedings 2, no. 20: 1289. https://doi.org/10.3390/proceedings2201289
APA StyleRokicka, M., Zieliński, M., Dębowski, M., & Głowacka, K. (2018). Microwave Heating Impact on the Oil Yield from Botryococcus braunii Algae Biomass. Proceedings, 2(20), 1289. https://doi.org/10.3390/proceedings2201289







