Removal of Diclofenac and Metformin from Water in Laboratory Photo Reactor †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Synthetic Solution and Experimental Procedure
2.3. Analytical Procedure
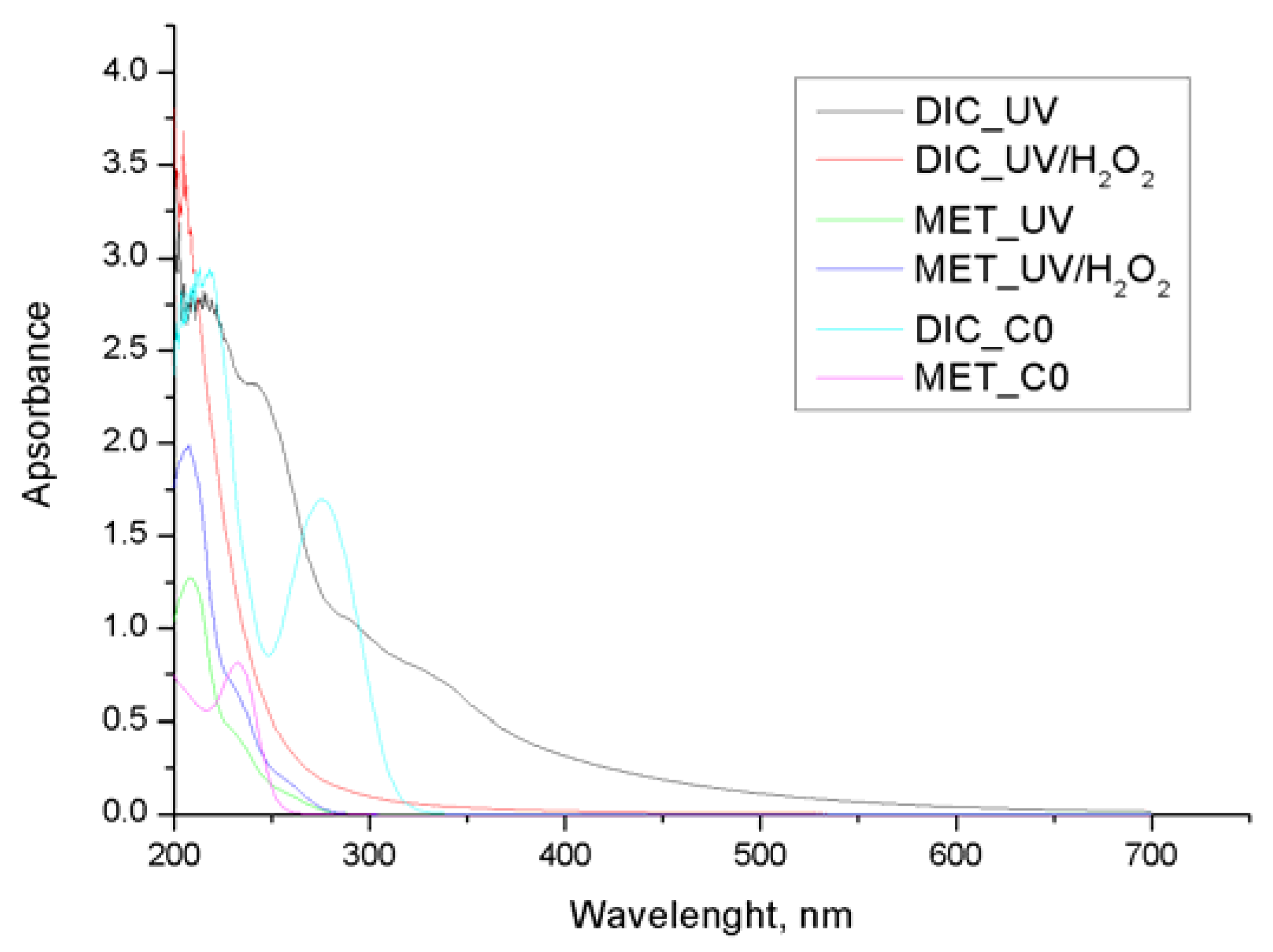
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bagheri, S.; TermehYousefi, A.; Do, T. Photocatalytic pathway toward degradation of environmental pharmaceuticals pollutants: Structure, kinetics and mechanism approach. Catal. Sci. Technol. 2017, 7, 4548–4569. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Liu, S.; Zeng, G.; Jinag, L.; Tan, X.; Zhou, L.; Zeng, W.; Li, T.; Yang, C. Adsorption of emerging contaminant metformin using graphene oxide. Chemospere 2017, 179, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Perisic, D.J.; Gilja, V.; Stankov, M.N.; Katancic, Z.; Kusic, H.; Stangar, U.L.; Dionysiou, D.D.; Bozic, A.L. Removal of diclofenac from water by zeolite-assisted advanced oxidation processes. J. Photochem. Photobiol. A 2016, 321, 238–247. [Google Scholar] [CrossRef]
- Briones, R.M.; Sarmah, A.K.; Padhye, L.P. A global perspective on the use, occurrence, fate and effects of antidiabetic drug metformin in natural and engineered ecosystems. Environ. Pollut. 2016, 219, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Safari, G.H.; Hosefini, M.; Seyedsalehi, M.; Kamani, H.; Jaafari, J.; Mahvi, A.H. Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int. J. Environ. Sci. Technol. 2015, 12, 603–616. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Toxicology Data Network. Available online: https://toxnet.nlm.nih.gov/ (accessed on 1 June 2018).
- ISO 11348-1: 2008 Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminiscent Bacteria Test); British Standards Institution: London, UK, 2008.
| Substance | Structure | Molecular weight (g/mol), Mw | Log Kow | Solubility in Water (mg/L), Sw |
|---|---|---|---|---|
| Diclofenac | 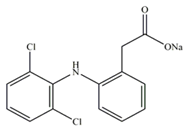 | 318 | 0.7 | 2430 |
| Metformin | 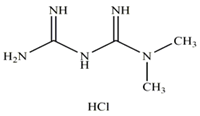 | 165 | −2.64 | 1.06 × 106 |
| Treatment | Removals (%) | |
|---|---|---|
| Diclofenac | Metformin | |
| UV | 30 | 50 |
| UV/H2O2 | 90 | 100 |
| Treatment | Toxicity inhibition (%) | |
|---|---|---|
| Diclofenac | Metformin | |
| Initial concentration, (C0) | 75 | 13 |
| UV | 33 | 38 |
| UV/H2O2 | 78 | 77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maćerak, A.L.; Kerkez, Đ.; Bečelić-Tomin, M.; Pilipović, D.T.; Kulić, A.; Jokić, J.; Dalmacija, B. Removal of Diclofenac and Metformin from Water in Laboratory Photo Reactor. Proceedings 2018, 2, 1288. https://doi.org/10.3390/proceedings2201288
Maćerak AL, Kerkez Đ, Bečelić-Tomin M, Pilipović DT, Kulić A, Jokić J, Dalmacija B. Removal of Diclofenac and Metformin from Water in Laboratory Photo Reactor. Proceedings. 2018; 2(20):1288. https://doi.org/10.3390/proceedings2201288
Chicago/Turabian StyleMaćerak, Anita Leovac, Đurđa Kerkez, Milena Bečelić-Tomin, Dragana Tomašević Pilipović, Aleksandra Kulić, Jovana Jokić, and Božo Dalmacija. 2018. "Removal of Diclofenac and Metformin from Water in Laboratory Photo Reactor" Proceedings 2, no. 20: 1288. https://doi.org/10.3390/proceedings2201288
APA StyleMaćerak, A. L., Kerkez, Đ., Bečelić-Tomin, M., Pilipović, D. T., Kulić, A., Jokić, J., & Dalmacija, B. (2018). Removal of Diclofenac and Metformin from Water in Laboratory Photo Reactor. Proceedings, 2(20), 1288. https://doi.org/10.3390/proceedings2201288





