Abstract
In this work gas sensing properties of Langmuir-Schaefer monolayer organic field-effect transistors (LS OFETs) prepared from organosilicon derivative of [1]benzothieno[3,2-b][1]-benzothiophene (BTBT) have been investigated. The monolayer has been deposited using Langmuir-Schaefer method, which results in a uniform low-defect monolayer with excellent electrical performance, hole mobility up to 7 × 10−2 cm2 V−1 s−1, the threshold voltage around 0 V and on-off ratio of 104. Developed sensors demonstrate a long-term stability of a half-year storage under ambient conditions. Preliminary investigations demonstrated that the LS OFETs give instantaneous response on ammonia and hydrogen sulfide at low concentrations. The results reported open new perspectives for the OFET-based gas-sensing technology.
1. Introduction
Organic field-effect transistors (OFETs) have garnered considerable attention because of their application in large-area, low cost and low power electronics [1]. The reliability of organic electronics devices still remains a crucial issue due to a strong sensitivity of organic semiconductors (OSCs) to chemical, optical and mechanical impacts, thus limiting the OFET technology commercialization as a competitor to traditional silicon electronics [2]. While such sensitivity is traditionally considered as a drawback, it simultaneously enables utilization of OFETs in various sensing applications, including chemical sensing. The number of OFET-based gas sensors reported up to date typically lack of air operation capability, selectivity to water and easily scalable fabrication technique [2,3].
In our work, we propose a self-assembled Langmuir-Schaefer monolayer field-effect transistor based on Langmuir-Schaefer monolayers of disiloxane derivative of benzothieno[3,2-b][1]benzothiophene (BTBT) (1,3-bis[11-(7-hexyl[1]benzothieno-[3,2-b][1]benzothien-2-yl)undecyl]-1,1,3,3-tetramethyldisiloxane) for chemical sensing (Figure 1) [4]. Selection of the semiconductor material is based on high charge carrier mobility demonstrated for BTBT molecules and high chemical stability of disiloxanes. The OSC monolayer has been deposited using Langmuir-Schaefer method. The conditions of uniform low-defect monolayer formation were found for this technique. Fabricated monolayers were used for preparation of OFET devices, which demonstrated excellent electrical performance with the hole mobility up to 7 × 10−2 cm2 V−1 s−1, the threshold voltage around 0 V and on-off ratio of 104 as well as long-term stability of a half-year storage under ambient conditions. We demonstrated that the monolayer OFETs give instantaneous response on ammonia and hydrogen sulfide at low concentrations (Limit Of Detection (LOD) down to tens ppb). These findings show a great potential of Langmuir-Schaefer monolayer OFETs for large-area sensing devices application.
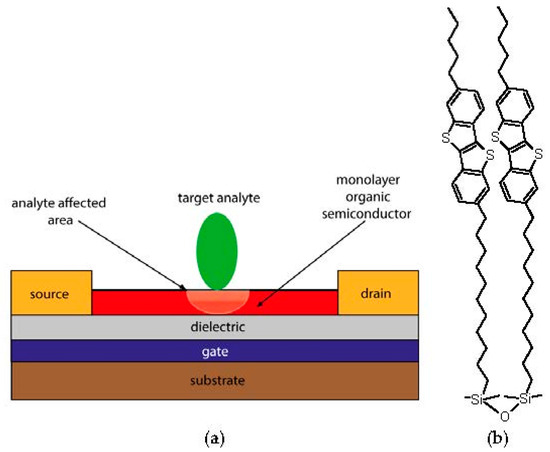
Figure 1.
Langmuir-Schaefer monolayer organic field-effect transistors (LS OFETs) as chemical sensors: (a) The illustration of the working principle. An extremely low thickness of LS film (2–3 nm) enables both ultrafast sensor response and low LOD; (b) Chemical structure of 1,3-bis[11-(7-hexyl[1]benzothieno-[3,2-b][1]benzothien-2-yl)undecyl]-1,1,3,3-tetramethyldisiloxane.
2. Materials and Methods
2.1. OFETs Substrates
LS OFETs were prepared on heavily doped silicon substrates with thermally grown oxide SiO2. The dielectric thickness was 200 nm, and its measured capacitance was 13 nF/cm2. Gold source and drain electrodes were thermally evaporated through a shadow mask. The resulting bottom gate bottom contact configuration had fixed channel size of W = 1000 μm and L = 30 μm.
2.2. Monolayer Deposition
The BTBT dimer has been synthesized as described before [4]. The monolayers have been deposited with Langmuir-Schaefer technique. The solution was prepared by dissolving the BTBT dimer in toluene (Acros, used as received) and spread on the water surface. Data were collected with a Nima 712BAM system equipped by a Brewster angle microscope MicroBAM2 using a Teflon trough and barriers at room temperature. The monolayers were compressed with the speed equal to 200 mm/min. LS films were obtained by horizontal transfer on silicon substrates, modified with octyldimethylchlorosilane (ODMS). Prior to electrical measurements the unwanted part of the OSC monolayer film was manually removed to prevent parasitic current. The LS OFETs fabricated were annealed at normal conditions during 72 h to remove water traces.
2.3. Sensor Properties
The sensors properties were tested in a specially designed Teflon chamber with a free volume of ~2.3 cm3. The LS OFET’s response to toxic gases have been evaluated in dry air flow containing known amounts of NH3 or H2S diluted with the carrier gas generated by zero air generator (Khimelectronica, Russia). Mixtures of 200–5000 ppb of ammonia and hydrogen sulfide in carrier gas were prepared using computer driven GGS-K generator (Monitoring, Russia). The calibrated permeation tubes (Monitoring, Russia) of NH3 and H2S were used. Mixtures of 10–200 ppb of toxic gases in dry air were prepared by additional diluting the GGS-K generator output with a synthetic air flow from a cylinder controlled by the mass-flow controller (Bronkhorst, The Netherlands). The electrical measurements were performed in a stabilized concentration that was reached after a 1 min flow.
3. Results and Discussion
The drain current (𝐼d) in a field-effect transistor in saturation mode can be described by the equation: [5]
where 𝐿 and 𝑊 are channel length and width respectively, is a gate voltage, is a dielectric capacitance per unit area. and are the threshold voltage and charge carrier mobility of the device respectively. During the exposure of the device to small amounts of NH3 or H2S in dry air with both gate and drain voltages were kept at constant biases of −40 V. In these conditions the drain current in the device permanently decays, and the decay rate was found to be dependent on toxic gas concentration. The decay was empirically characterized by stretched exponent behavior:
where 𝛽 is a dispersion coefficient, dependent on the temperature, 𝜏—characteristic relaxation time dependent on the toxic gas concentration. Typical dynamic response of the LS OFET sensor to 200 ppb H2S is shown on Figure 2a. The decay rate in dry air (“zero” decay) should be attributed to bias stress effects in OFET. When H2S is introduced into the cell, “zero” decay is combined with an extra effect of the toxic gas presence resulting in a current decay rate variation. As the toxic gas flow is turned off, the current slowly restores to the initial value. However, being affected by the gate bias stress, it continues to decrease. However, the device fully restores to the initial values after being kept dry air for 30–40 min without the applied voltage. The devices investigated demonstrate half-year storage under ambient conditions, which is a remarkable result for OFET-based chemical sensors.
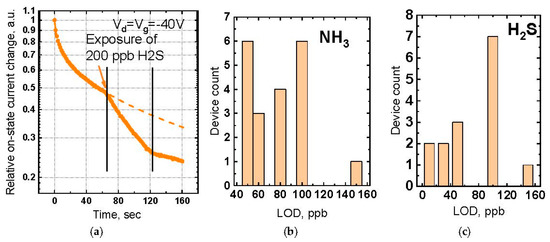
Figure 2.
Sensor properties of LS OFETs: (a) Dynamic response of the sensor to 200 ppb H2S; (b) The statistical distribution of LOD to NH3; (c) The statistical distribution of LOD to H2S.
Figure 2b,c show the statistical distribution of LOD to NH3 and H2S correspondingly. The LS OFETs show high LOD reproducibility due to conditions of uniform low-defect monolayer. The lowest LOD values for NH3 and H2S are 50 and 10 ppb correspondingly, which are among the best values reported for OFET gas sensors.
4. Conclusions and Outlook
To sum up, the sensing properties of Langmuir-Schaefer monolayer organic field-effect transistors (LS OFETs) prepared from organosilicon derivative of [1]benzothieno[3,2-b][1]-benzothiophene (BTBT) have been investigated. The monolayer has been deposited using Langmuir-Schaefer method, which results in a uniform low-defect monolayer with excellent electrical performance, hole mobility up to 7 × 10−2 cm2 V−1 s−1, the threshold voltage around 0 V and on-off ratio of 104. Developed sensors demonstrate a long-term stability of a half-year storage under ambient conditions. We demonstrated that the LS OFETs give instantaneous response on ammonia and hydrogen sulfide at low concentrations (LOD down to tens ppb). The work to correlate the variation of the OFET’s parameters with the analyte type is currently in progress. The results reported open new perspectives for the OFET-based gas-sensing technology.
Author Contributions
E.A. and A.S. conceived and designed the experiments; O.B. synthesized the BTBT derivative; V.C. prepared sensor samples; A.T. performed the experiments and processed the data; S.P. and A.V. supervised the research; A.S. wrote the paper.
Acknowledgments
This work was performed in the framework of leading science school NSh-5698.2018.3. Authors are also grateful to RFBR (grants 17-03-00222 and 18-33-01210) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sirringhaus, H. 25th anniversary article: Organic field-effect transistors: The path beyond amorphous silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, P.A.; Sharma, A.; Mathijssen, S.G.J.; Kemerink, M.; de Leeuw, D.M. Operational stability of organic field-effect transistors. Adv. Mater. 2012, 24, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Andringa, A.M.; Spijkman, M.J.; Smits, E.C.P.; Mathijssen, S.G.J.; van Hal, P.A.; Setayesh, S.; Willard, N.P.; Borshchev, O.V.; Ponomarenko, S.A.; Blom, P.W.M.; et al. Gas sensing with self-assembled monolayer field-effect transistors. Org. Electron. 2010, 11, 895–898. [Google Scholar] [CrossRef]
- Borshchev, O.V.; Sizov, A.S.; Agina, E.V.; Bessonov, A.A.; Ponomarenko, S.A. Synthesis of organosilicon derivatives of [1]benzothieno[3,2-b][1]-benzothiophene for efficient monolayer langmuir-blodgett organic field effect transistors. Chem. Commun. 2017, 53, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, G. Organic field-effect transistors. Adv. Mater. 1998, 10, 365–377. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).