Chemiresistors Based on Bisdithiocarbamate Interlinked Gold Nanoparticles †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
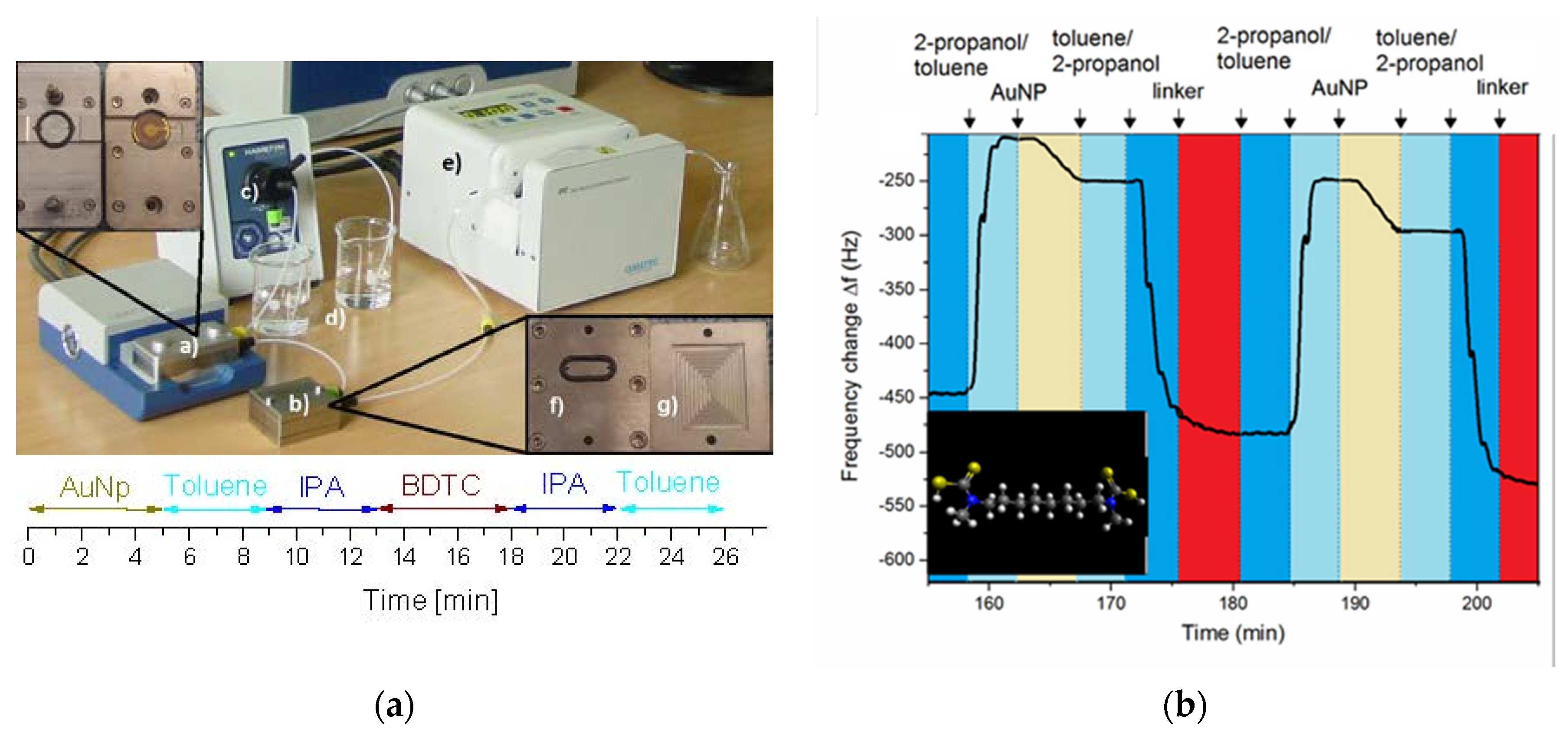
3.1. Layer-by-Layer Self-Assembly
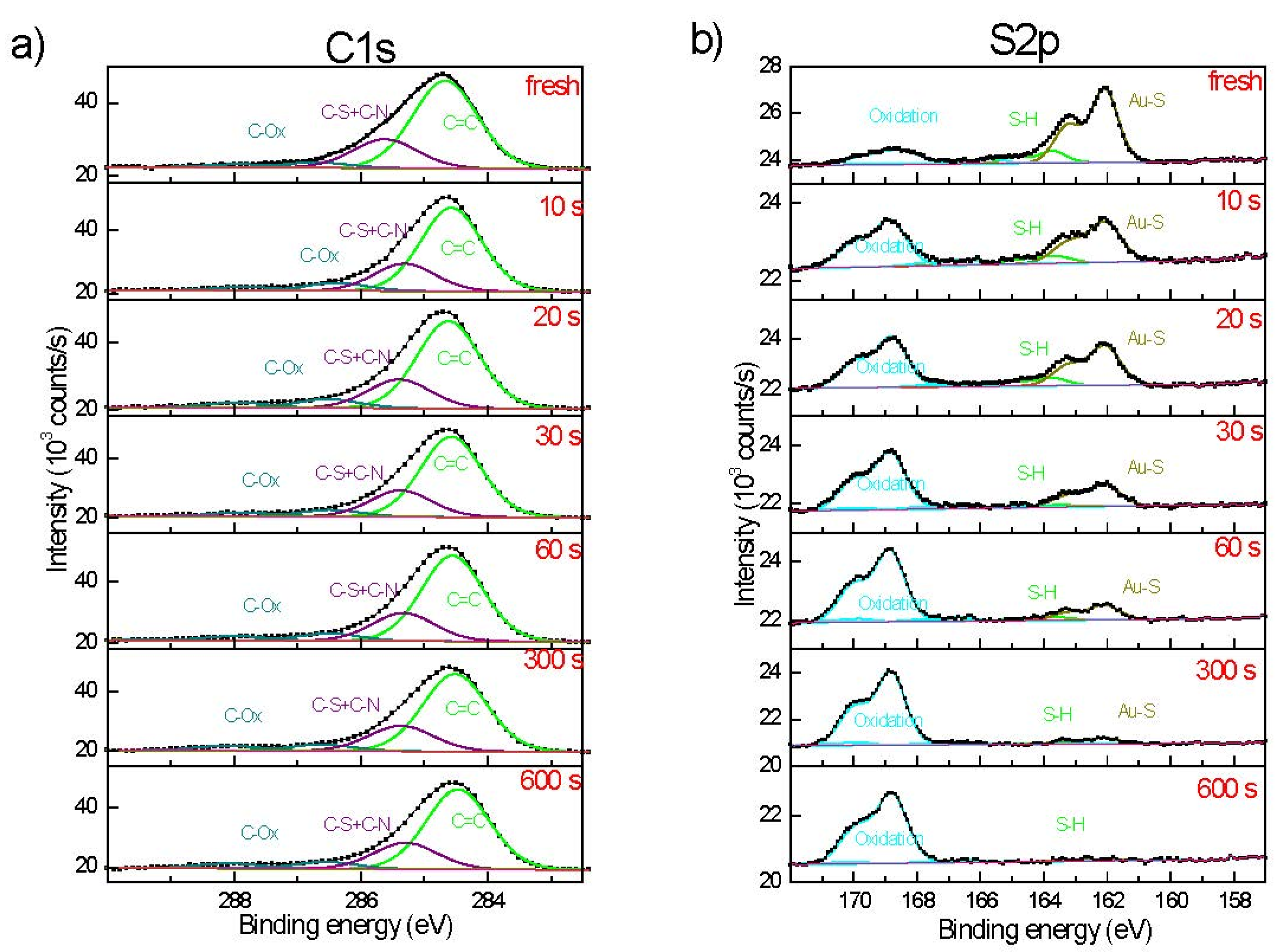
3.2. Composite Charactzerisation
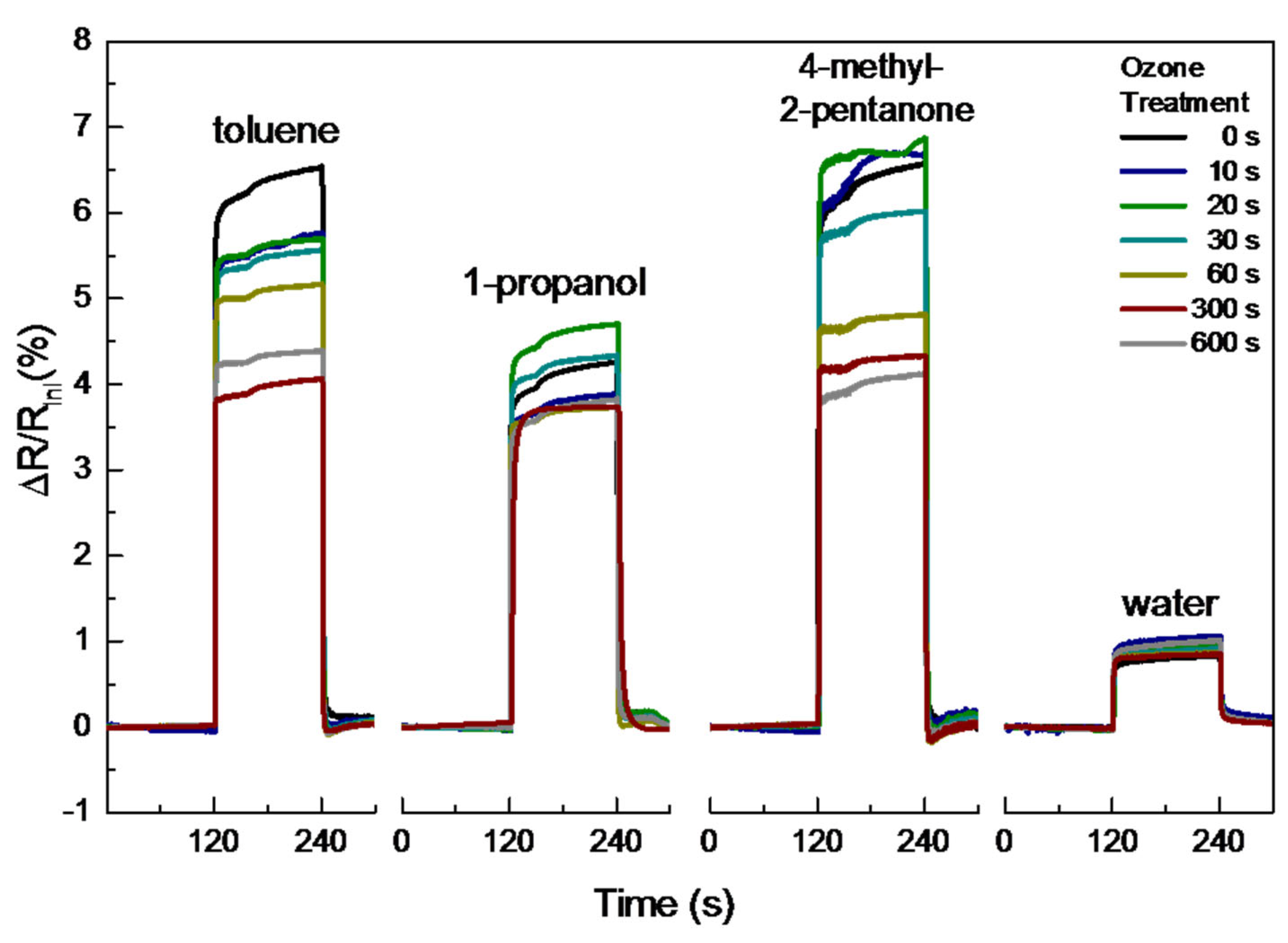
3.3. VOC Sensing
4. Conclusions and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Joseph, Y.; Besnard, I.; Rosenberger, M.; Guse, B.; Nothofer, H.-G.; Wessels, J.M.; Wild, U.; Knop-Gericke, A.; Su, D.; Schlögl, R.; et al. Self-Assembled Gold Nanoparticle/Alkanedithiol Films: Preparation, Electron Microscopy, XPS-Analysis, Charge Transport, and Vapor-Sensing Properties. J. Phys. Chem. B 2003, 107, 7406–7413. [Google Scholar] [CrossRef]
- Joseph, Y.; Guse, B.; Nelles, G. Aging of 1,ω-Alkyldithiol Interlinked Au Nanoparticle Networks. Chem. Mater. 2009, 21, 1670–1676. [Google Scholar] [CrossRef]
- Daskal, Y.; Tauchnitz, T.; Güth, F.; Dittrich, R.; Joseph, Y. Assembly Behavior of Organically Interlinked Gold Nanoparticle Composite Films: A Quartz Crystal Microbalance Investigation. Langmuir 2017, 33, 11869–11877. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskal, Y.; Rabe, S.; Dittrich, R.; Oestreich, C.; Joseph, Y. Chemiresistors Based on Bisdithiocarbamate Interlinked Gold Nanoparticles. Proceedings 2018, 2, 933. https://doi.org/10.3390/proceedings2130933
Daskal Y, Rabe S, Dittrich R, Oestreich C, Joseph Y. Chemiresistors Based on Bisdithiocarbamate Interlinked Gold Nanoparticles. Proceedings. 2018; 2(13):933. https://doi.org/10.3390/proceedings2130933
Chicago/Turabian StyleDaskal, Yelyena, Susann Rabe, Rosemarie Dittrich, Christiane Oestreich, and Yvonne Joseph. 2018. "Chemiresistors Based on Bisdithiocarbamate Interlinked Gold Nanoparticles" Proceedings 2, no. 13: 933. https://doi.org/10.3390/proceedings2130933
APA StyleDaskal, Y., Rabe, S., Dittrich, R., Oestreich, C., & Joseph, Y. (2018). Chemiresistors Based on Bisdithiocarbamate Interlinked Gold Nanoparticles. Proceedings, 2(13), 933. https://doi.org/10.3390/proceedings2130933




