Fast Formation of Lipid Bilayer Membranes for Simultaneous Analysis of Molecular Transport Using Parylene Coated Chips †
Abstract
:1. Introduction
2. Materials and Methods
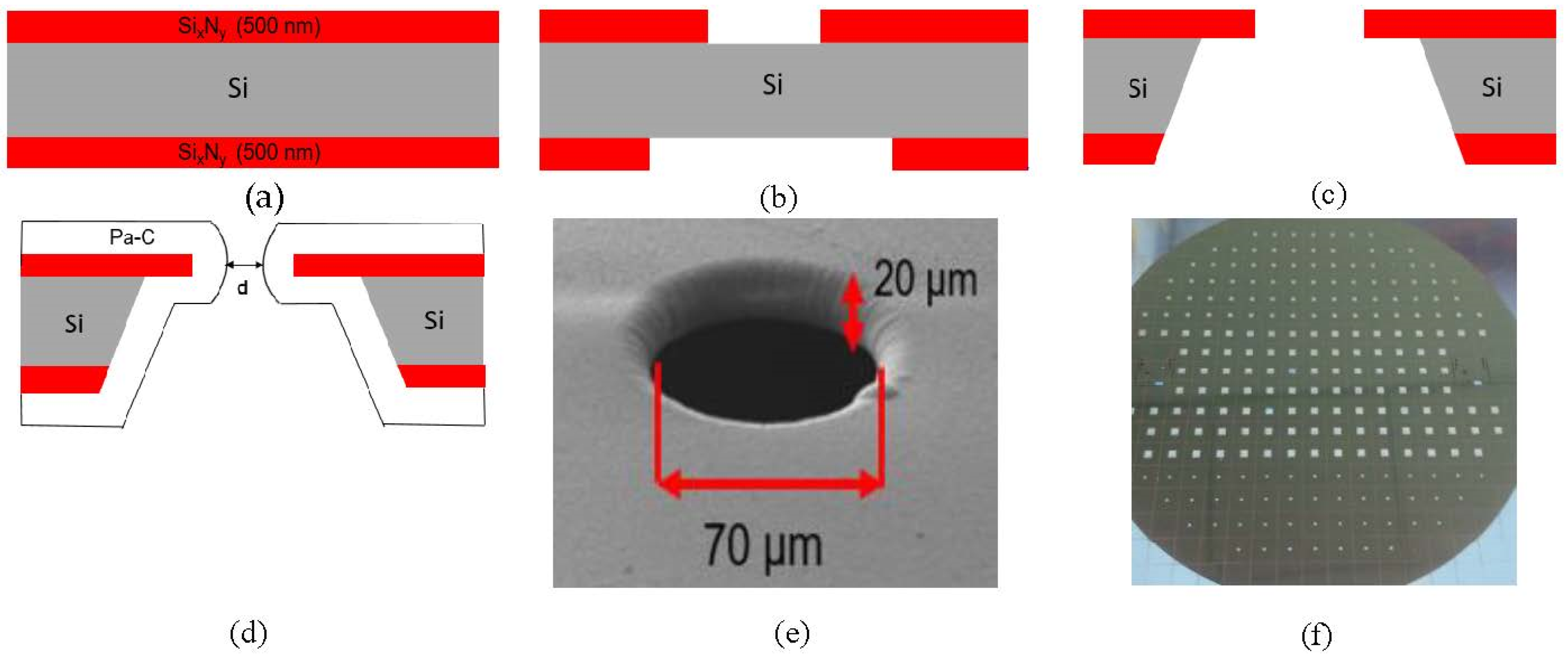
2.1. Lipid Bilayer Chip Fabrication
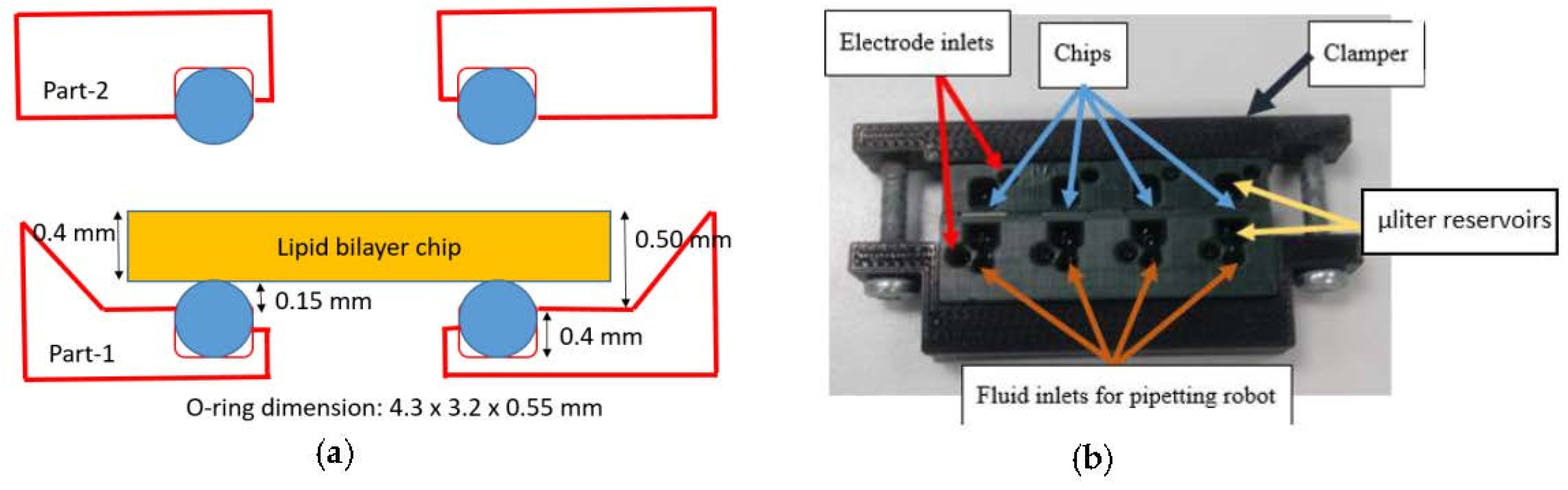
2.2. Designing and Printing the Parallel Bilayer Analysis Platform
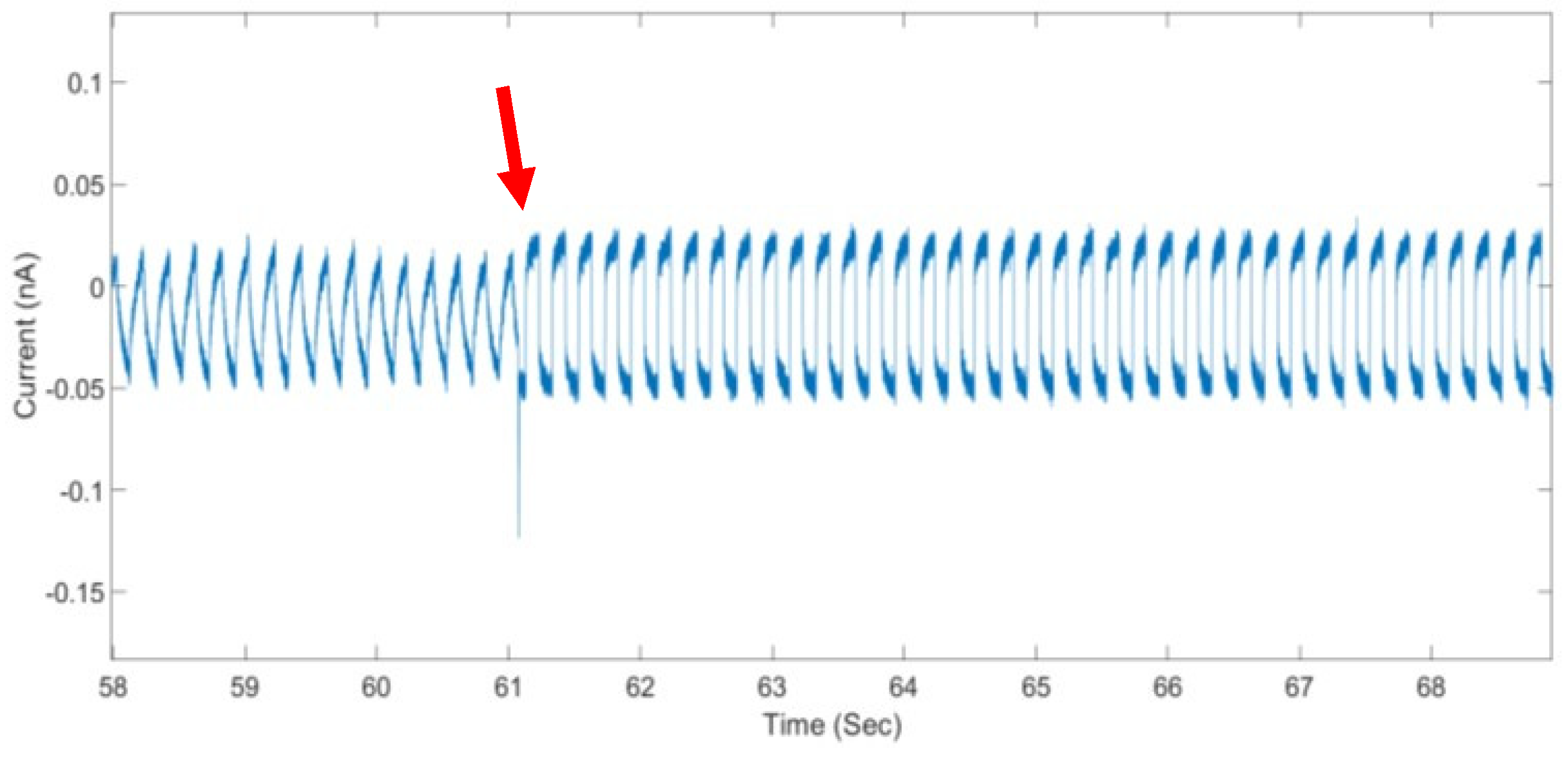
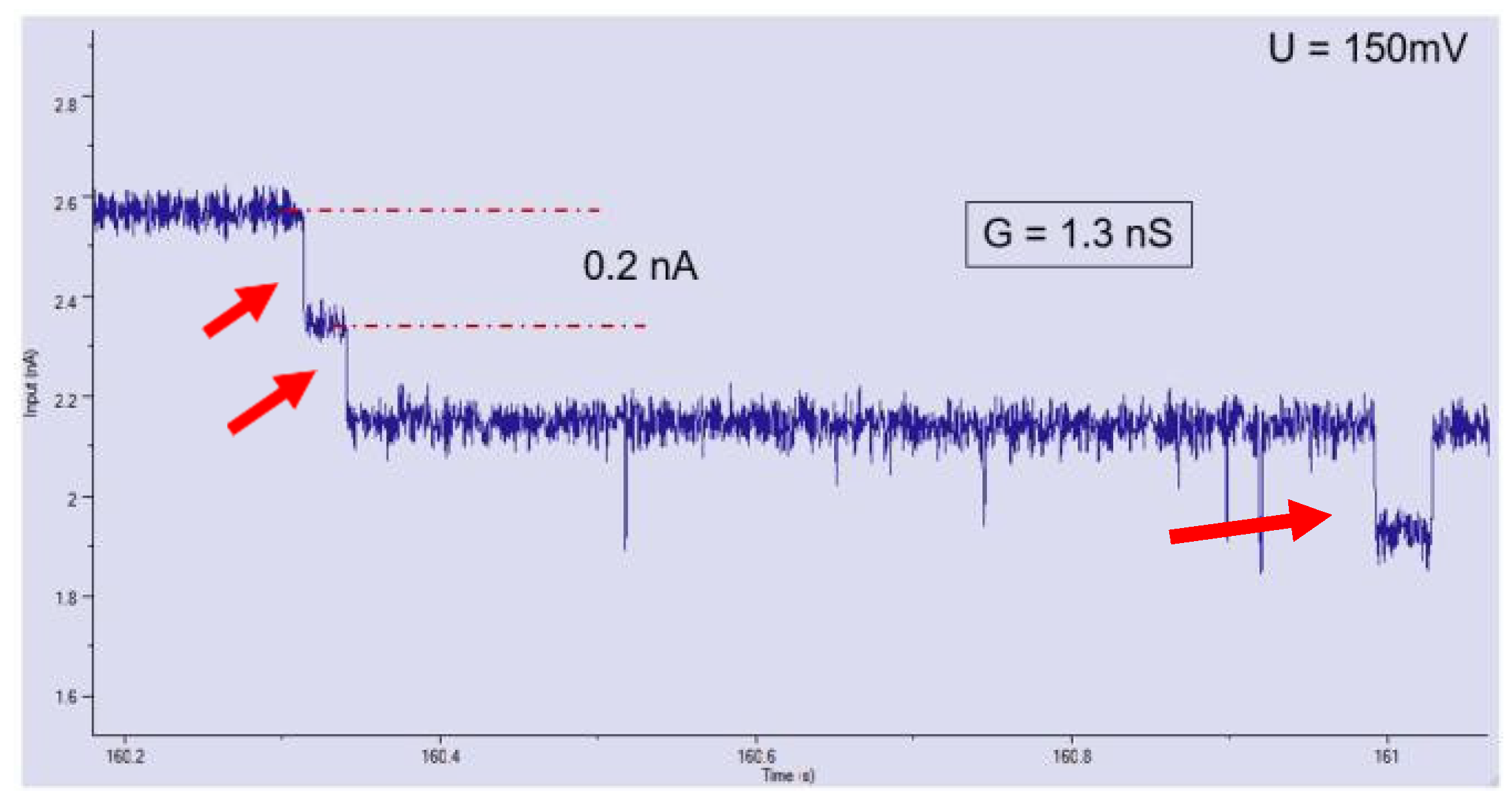
3. Results
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mayer, M.; Kriebel, J.K.; Tosteson, M.T.; Whitesides, G.M. Microfabricated teflon membranes for low-noise recordings of ion channels in planar lipid bilayers. Biophys. J. 2003, 85, 2684–2695. [Google Scholar] [CrossRef]
- O’Shaughnessy, T.J.; Hu, J.E.; Kulp, J.L.; Daly, S.M.; Ligler, F.S. Laser ablation of micropores for formation of artificial planar lipid bilayers. Biomed. Microdevices 2007, 9, 863–868. [Google Scholar] [CrossRef] [PubMed]
- van den Driesche, S.; Lucklum, F.; Bunge, F.; Vellekoop, M.J. 3D printing solutions for microfluidic chip-to-world connections. Micromachines 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Danelon, C.; Suenaga, A.; Winterhalter, M.; Yamato, I. Molecular Origin of the Cation Selectivity in OmpF Porin. Single Channel Conductances versus Free Energy Calculation. Biophys. Chem. 2003, 104, 591–603. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.; Driesche, S.v.d.; Oellers, M.; Hemmler, R.; Gall, K.; Bhamidimarri, S.P.; Winterhalter, M.; Wagner, R.; Vellekoop, M.J. Fast Formation of Lipid Bilayer Membranes for Simultaneous Analysis of Molecular Transport Using Parylene Coated Chips. Proceedings 2018, 2, 920. https://doi.org/10.3390/proceedings2130920
Ahmed T, Driesche Svd, Oellers M, Hemmler R, Gall K, Bhamidimarri SP, Winterhalter M, Wagner R, Vellekoop MJ. Fast Formation of Lipid Bilayer Membranes for Simultaneous Analysis of Molecular Transport Using Parylene Coated Chips. Proceedings. 2018; 2(13):920. https://doi.org/10.3390/proceedings2130920
Chicago/Turabian StyleAhmed, Tanzir, Sander van den Driesche, Martin Oellers, Roland Hemmler, Karsten Gall, Satya Prathyusha Bhamidimarri, Mathias Winterhalter, Richard Wagner, and Michael J. Vellekoop. 2018. "Fast Formation of Lipid Bilayer Membranes for Simultaneous Analysis of Molecular Transport Using Parylene Coated Chips" Proceedings 2, no. 13: 920. https://doi.org/10.3390/proceedings2130920
APA StyleAhmed, T., Driesche, S. v. d., Oellers, M., Hemmler, R., Gall, K., Bhamidimarri, S. P., Winterhalter, M., Wagner, R., & Vellekoop, M. J. (2018). Fast Formation of Lipid Bilayer Membranes for Simultaneous Analysis of Molecular Transport Using Parylene Coated Chips. Proceedings, 2(13), 920. https://doi.org/10.3390/proceedings2130920




