Fabrication of SnO2 Flexible Sensor by Inkjet Printing Technology †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sol Preparation
2.2. Ink Preparation
2.3. Contact Electrodes on Foil
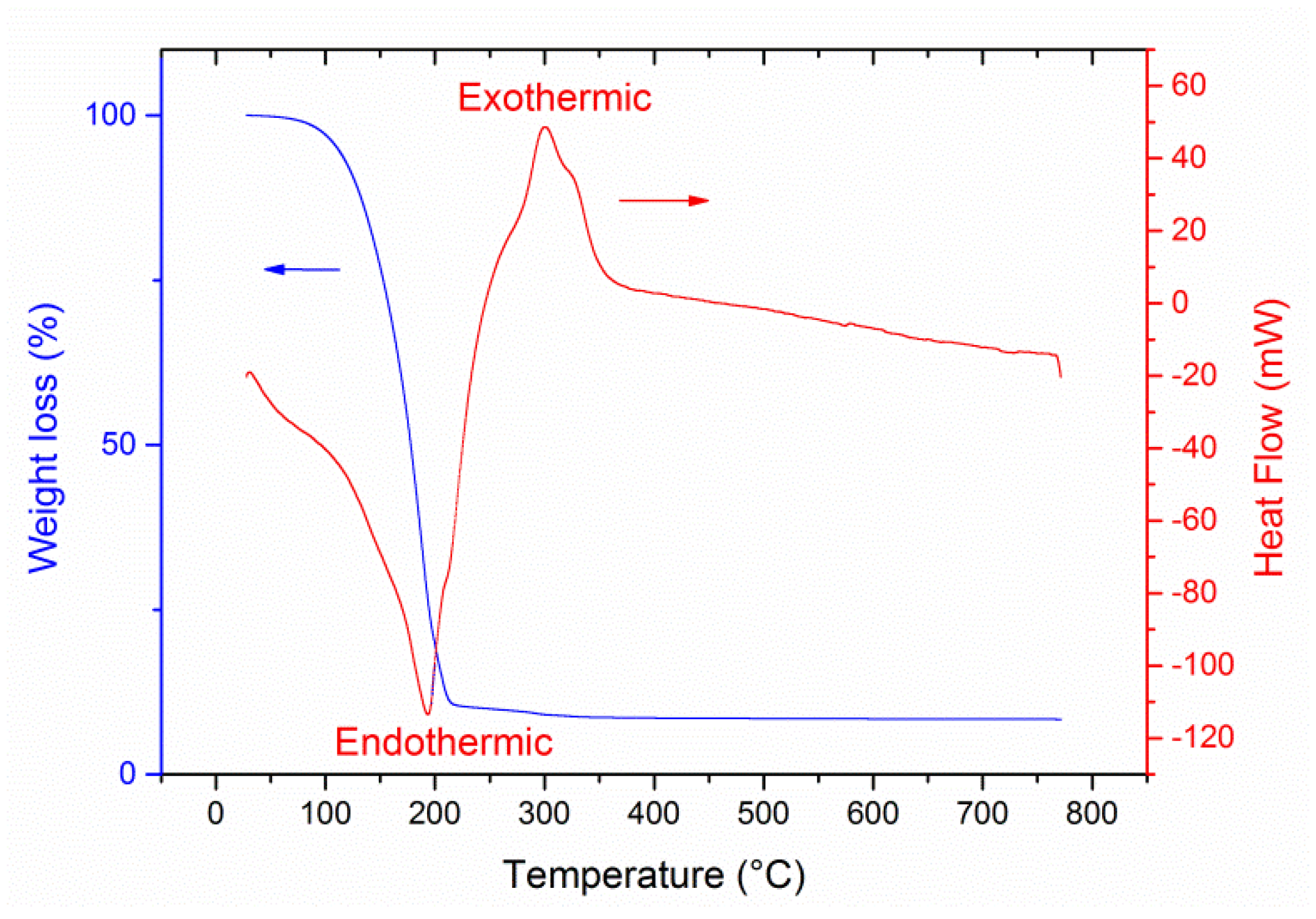
2.4. Characterization
3. Results
4. Conclusions
Conflicts of Interest
References
- Yeo, J.C.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 16043. [Google Scholar]
- Luo, W.; Deng, J.; Fu, Q.; Zhou, D.; Hu, Y.; Gong, S.; Zheng, Z. Nanocrystalline SnO2 film prepared by the aqueous sol-gel method and its application as sensing films of the resistance and SAW H2S sensor. Sens. Actuators B Chem. 2015, 217, 119–128. [Google Scholar] [CrossRef]
- Köse, H.; Karaal, Ş.; Aydin, A.O.; Akbulut, H. Structural properties of size-controlled SnO2 nanopowders produced by sol-gel method. Mater. Sci. Semicond. Process. 2015, 38, 404–412. [Google Scholar] [CrossRef]
- Derby, B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully inkjet printed SnO2 gas sensor on plastic substrate. Sens. Actuators B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef]
- Tamaki, J.; Miyaji, A.; Makinodan, J.; Ogura, S.; Konishi, S. Effect of micro-gap electrode on detection of dilute NO2 using WO3 thin film microsensors. Sens. Actuators B Chem. 2005, 108, 202–206. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassem, O.; Saadaoui, M.; Rieu, M.; Viricelle, J.P. Fabrication of SnO2 Flexible Sensor by Inkjet Printing Technology. Proceedings 2018, 2, 907. https://doi.org/10.3390/proceedings2130907
Kassem O, Saadaoui M, Rieu M, Viricelle JP. Fabrication of SnO2 Flexible Sensor by Inkjet Printing Technology. Proceedings. 2018; 2(13):907. https://doi.org/10.3390/proceedings2130907
Chicago/Turabian StyleKassem, Omar, Mohamed Saadaoui, Mathilde Rieu, and Jean Paul Viricelle. 2018. "Fabrication of SnO2 Flexible Sensor by Inkjet Printing Technology" Proceedings 2, no. 13: 907. https://doi.org/10.3390/proceedings2130907
APA StyleKassem, O., Saadaoui, M., Rieu, M., & Viricelle, J. P. (2018). Fabrication of SnO2 Flexible Sensor by Inkjet Printing Technology. Proceedings, 2(13), 907. https://doi.org/10.3390/proceedings2130907



