Synthesis of Fluorescein Aldehydes for the Sensitive Detection of L-Cysteine †
Abstract
1. Introduction
2. Materials and Methods
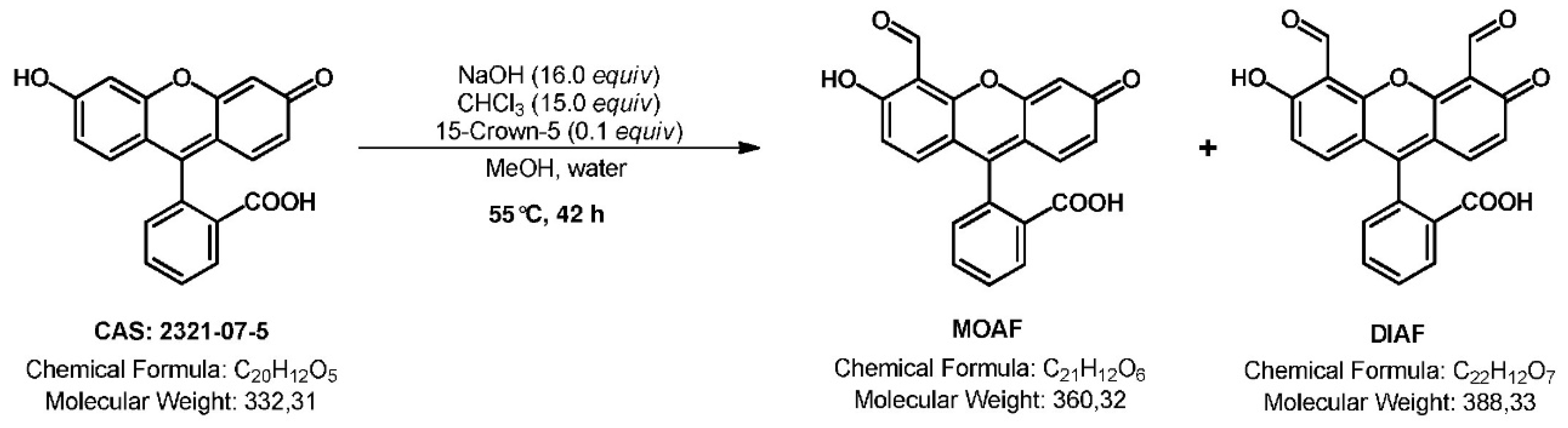
2.1. Chemical Synthesis
2.2. Cys Measurements
3. Results and Discussion
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Kim, H.J. Fluorescein aldehyde with disulfide functionality as a fluorescence turn-on probe for cysteine and homocysteine in HEPES buffer. Org. Biomol. Chem. 2013, 11, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rusin, O.; Xu, X.; Kim, K.K.; Escobedo, J.O.; Fakayode, S.O.; Fletcher, K.A.; Lowry, M.; Schowalter, C.M.; Lawrence, C.M.; et al. Detection of homocysteine and cysteine J. Am. Chem. Soc. 2005, 127, 15949–15958. [Google Scholar] [CrossRef] [PubMed]
- Ewann, F.; Hoffman, P.S. Cysteine metabolism in Legionella pneumophilia: Characterization of an L-Cystine-utilizing mutant. Appl. Environ. Microb. 2006, 72, 3993–4000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rusin, O.; St. Luce, N.N.; Agbaria, R.A.; Escobedo, J.O.; Jiang, S.; Warner, I.M.; Dawan, F.B.; Lian, K.; Strongin, R.M. Visual Detection of Cysteine and Homocysteine. J. Am. Chem. Soc. 2004, 126, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent sensors for Zn2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettenauer, J.; Semak, V.; Brandl, M. Synthesis of Fluorescein Aldehydes for the Sensitive Detection of L-Cysteine. Proceedings 2018, 2, 895. https://doi.org/10.3390/proceedings2130895
Ettenauer J, Semak V, Brandl M. Synthesis of Fluorescein Aldehydes for the Sensitive Detection of L-Cysteine. Proceedings. 2018; 2(13):895. https://doi.org/10.3390/proceedings2130895
Chicago/Turabian StyleEttenauer, Jörg, Vladislav Semak, and Martin Brandl. 2018. "Synthesis of Fluorescein Aldehydes for the Sensitive Detection of L-Cysteine" Proceedings 2, no. 13: 895. https://doi.org/10.3390/proceedings2130895
APA StyleEttenauer, J., Semak, V., & Brandl, M. (2018). Synthesis of Fluorescein Aldehydes for the Sensitive Detection of L-Cysteine. Proceedings, 2(13), 895. https://doi.org/10.3390/proceedings2130895




