Highly Sensitive NH3 Sensors Using CVD and Epitaxial Graphene Functionalised with Vanadium(V) Oxide: A Comparative Study †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
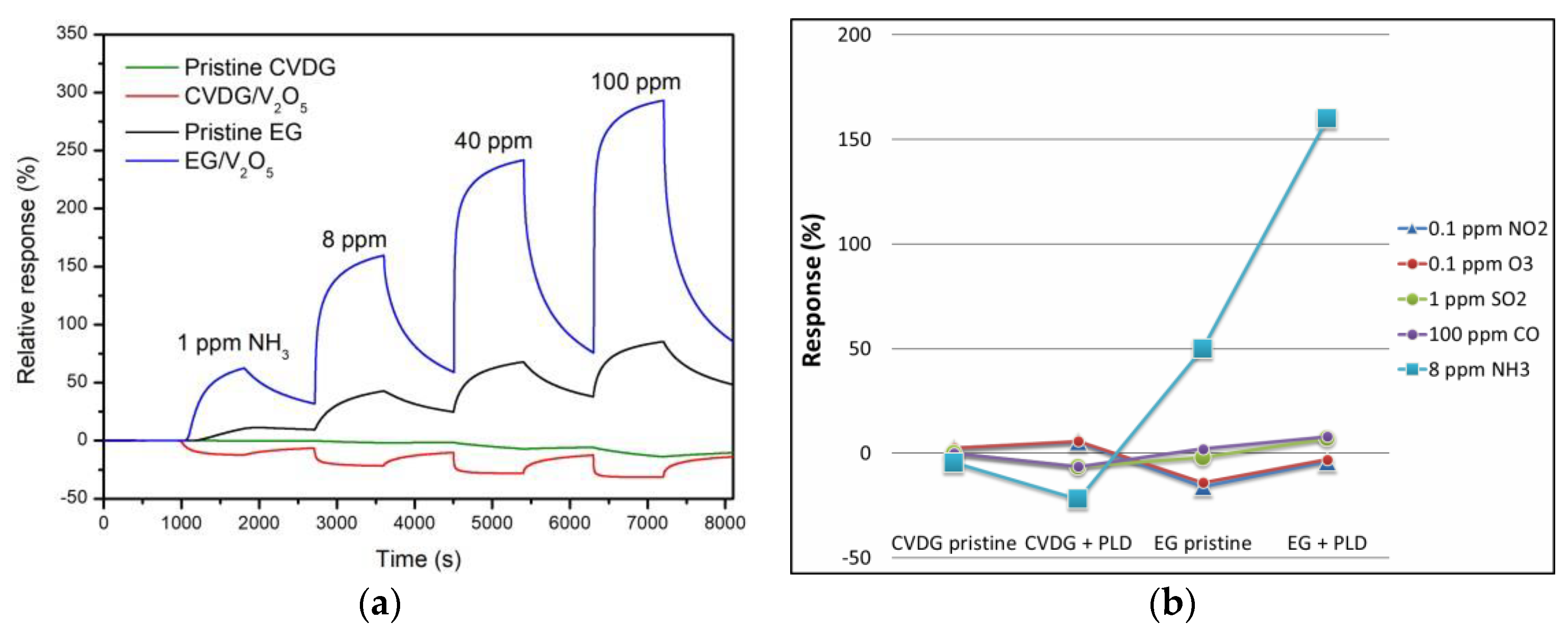
- Adsorption sites with comparable affinity constants b1 and b2 on both types of graphene indicate the similar effect of deposited V2O5 nanophase on the surface.
- Both transduction coefficients α1 and α2 are much smaller in case of CVD graphene sensor, which means less influence of gas adsorption on relative conductivity, and hence, lower relative gas response of the CVD graphene sensor. Taking into account that the gas adsorption mainly modifies the charge carrier concentration rather than mobility [1], the substantially larger response of EG sensor is probably arising from lower initial carrier doping of EG as compared to free electron concentration in CVD graphene.
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Lee, G.; Yang, G.; Cho, A.; Han, J.W.; Kim, J. Defect-engineered graphene chemical sensors with ultrahigh sensitivity. Phys. Chem. Chem. Phys. 2016, 18, 14198–14204. [Google Scholar] [CrossRef]
- Kodu, M.; Berholts, A.; Kahro, T.; Kook, M.; Ritslaid, P.; Seemen, H.; Avarmaa, T.; Alles, H.; Jaaniso, R. Graphene functionalised by laser-ablated V2O5 for a highly sensitive NH3 sensor. Beilstein J. Nanotechnol. 2017, 8, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Jaaniso, R.; Tan, O.K. Semiconductor Gas Sensors; Woodhead Publishing: Cambridge, UK, 2013; p. 147. [Google Scholar]
- Yakimova, R.; Virojanadara, C.; Gogova, D.; Syväjärvi, M.; Siche, D.; Larsson, K.; Johansson, L.I. Analysis of the formation conditions for large area epitaxial graphene on SiC substrates. Mater. Sci. Forum 2010, 645–648, 565–568. [Google Scholar] [CrossRef]
- Berholts, A.; Kahro, T.; Floren, A.; Alles, H.; Jaaniso, R. Photo-activated oxygen sensitivity of graphene at room temperature. Appl. Phys. Lett. 2014, 105, 163111. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Q.; Liu, Z.; Gui, G.; Huang, Z. Adsorption and oxidation of NH3 over V2O5/AC surface. Appl. Catal. B 2009, 92, 462–467. [Google Scholar] [CrossRef]
- Eriksson, J.; Puglisi, D.; Kang, Y.H.; Yakimova, R.; Lloyd Spetz, A. Adjusting the electronic properties and gas reactivity of epitaxial graphene by thin surface metallization. Phys. B Condens. Matter 2014, 439, 105–108. [Google Scholar] [CrossRef]
- Kodu, M.; Berholts, A.; Kahro, T.; Avarmaa, T.; Kasikov, A.; Niilisk, A.; Alles, H.; Jaaniso, R. Highly sensitive NO2 sensors by pulsed laser deposition on graphene. Appl. Phys. Lett. 2016, 109, 113108. [Google Scholar] [CrossRef]
- Jaaniso, R.; Kahro, T.; Kozlova, J.; Aarik, J.; Aarik, L.; Alles, H.; Floren, A.; Gerst, A.; Kasikov, A.; Niilisk, A.; et al. Temperature induced inversion of oxygen response in CVD graphene on SiO2. Sens. Actuators B Chem. 2014, 190, 1006–1013. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodu, M.; Berholts, A.; Kahro, T.; Eriksson, J.; Yakimova, R.; Avarmaa, T.; Renge, I.; Alles, H.; Jaaniso, R. Highly Sensitive NH3 Sensors Using CVD and Epitaxial Graphene Functionalised with Vanadium(V) Oxide: A Comparative Study. Proceedings 2018, 2, 854. https://doi.org/10.3390/proceedings2130854
Kodu M, Berholts A, Kahro T, Eriksson J, Yakimova R, Avarmaa T, Renge I, Alles H, Jaaniso R. Highly Sensitive NH3 Sensors Using CVD and Epitaxial Graphene Functionalised with Vanadium(V) Oxide: A Comparative Study. Proceedings. 2018; 2(13):854. https://doi.org/10.3390/proceedings2130854
Chicago/Turabian StyleKodu, Margus, Artjom Berholts, Tauno Kahro, Jens Eriksson, Rositsa Yakimova, Tea Avarmaa, Indrek Renge, Harry Alles, and Raivo Jaaniso. 2018. "Highly Sensitive NH3 Sensors Using CVD and Epitaxial Graphene Functionalised with Vanadium(V) Oxide: A Comparative Study" Proceedings 2, no. 13: 854. https://doi.org/10.3390/proceedings2130854
APA StyleKodu, M., Berholts, A., Kahro, T., Eriksson, J., Yakimova, R., Avarmaa, T., Renge, I., Alles, H., & Jaaniso, R. (2018). Highly Sensitive NH3 Sensors Using CVD and Epitaxial Graphene Functionalised with Vanadium(V) Oxide: A Comparative Study. Proceedings, 2(13), 854. https://doi.org/10.3390/proceedings2130854



