Abstract
Monitoring of specific biomarker is critical for determining progression of a disease or efficacy of a treatment. Currently, the standard for assessing amount of specific biomarkers is the enzyme linked immunosorbent assay (ELISA), which measures quantities on the nanogram scale. However, ELISA has high material costs, long incubation periods, requires large volume of samples and involves special instruments, which necessitates clinical samples to be sent to a lab. In order to provide rapid, accurate, easy, point-of-care measurement of biomarkers, electrochemical immunosensor can be used to provide specific and sensitive biomarker detection. Immunosensor allow near real-time results, reduced costs, and simple assays with no labelling. In this work, we developed an electrochemical biosensor to measure total immunoglobulin E (IgE), a marker of asthma disease that varies with age, gender, and disease in concentrations from 0.3–1000 ng/mL with consuming 20 µL volume of real blood sample. Also, sequential monitoring of total IgE with OVA induced mice is another application of this work and this sensor is an alternative approach for recording data and a more effective assay for understanding the cytotoxic effects of toxic materials.
1. Introduction
Recently, allergic disease is a major issue for the prevalence of allergic and asthma increasing steadily worldwide [1,2,3]. Immunoglobulin E (IgE) and cytokine detection is necessary for diagnosis of these types of disease related with inflammation, immunology, atherosclerosis. However, monitoring of these proteins has some limitations related to their low concentrations in biological samples so difficult obtaining correct analytical results [4]. Moreover, conventional analytical system such as ELISA requires large volume of samples, expensive and physically large instruments to analyze samples. There is need for a rapid, accurate, easy to use and inexpensive analysis of biomarker quantification. In this work, we developed a one of important biomarker for diagnosis of allergic response, total IgE electrochemical (EC) sensor using a multichannel screen-printed electrode with high sensitivity, selectivity and requiring only 20 µL of per sample based on amperometric analysis.
2. Materials and Methods
2.1. Electrode and Amperometric Detection
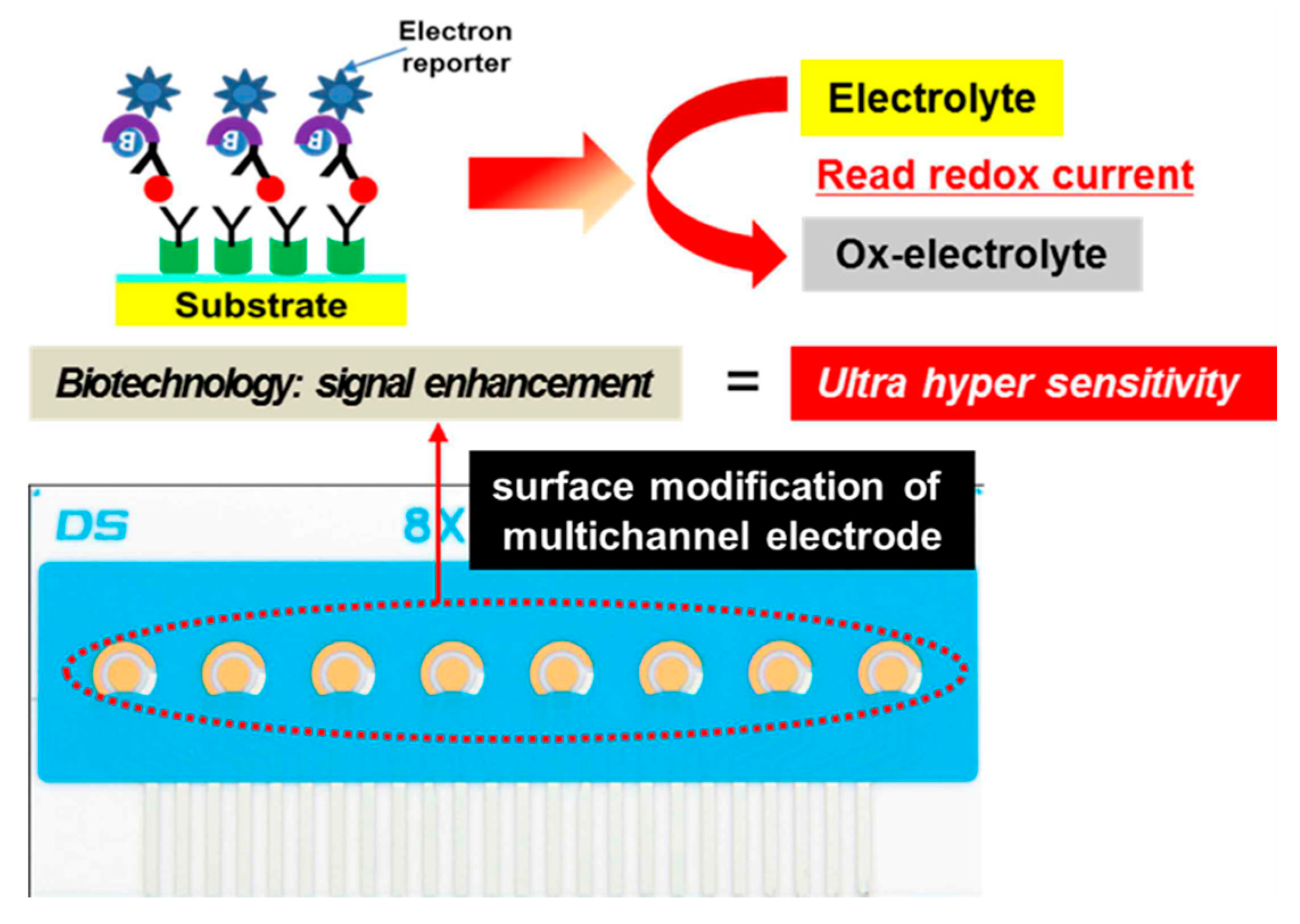
The fabrication of the immunosensor is conceptually represented in Figure 1. The DropSens (DropSens, Austurius, Spain) 220 AT gold electrode surface was modified through the addition of a self-assembled monolayer (SAM) containing a streptavidin bioconjugation. Utilizing the biotin-streptavidin interaction, we tethered biotinylated anti-ALP antibodies to the surface, creating an immunolayer specific for IgE. Chronoamperometry with applying 100 mV potential was performed in TMB (3,3,5,5,-tetramethylebenzdine) as an electrolyte solution at room temperature using electrochemical analyzer.
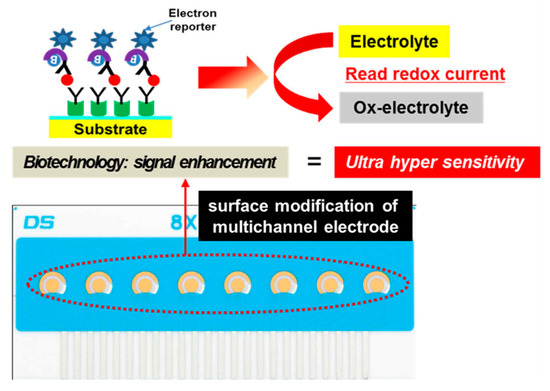
Figure 1.
Electrochemical measurement of total IgE based on multichannel screen-printed electrode.
2.2. In Vivo Studies
Six-week-old female BALB/c were purchased from Orient Bio Inc. (Seongnam, Korea). The mice were housed in an environmentally controlled animal room which was maintained at a temperature of 22 ± 3 °C, a relative humidity of 50 ± 20%, and an air ventilation rate of 10–20 changes/h with a 12 h light/dark cycle. Sterile pelleted food for experimental animals (PM Nutrition International, Richmond, VA, USA) and UV-irradiated (Steritron SX-1; Daeyoung, Inc., Incheon, Korea) and filtered (1 µm) tap water were provided. The mice were acclimatized for 6 days. All experiments were approved by the Institutional Animal Care and Use Committee of Korea Institute of Toxicology (KIT) and conducted according to the guidelines of Association for Assessment and Accreditation of Laboratory Animal Care International.
For OVA-induced asthma model, mice were intraperitoneally sensitized on days 1 and 8 with 20 µg of ovalbumin (OVA, Sigma-Aldrich, St Louis, MO, USA) emulsified in 1 mg of aluminum hydroxide (Thermo Fisher Scientific, Rockford, IL, USA) in a total volume of 200 µL. On days 17, 18, and 19 after initial sensitization with OVA, the mice were inhaled to aerosolized 5 % (v/v) OVA for 30 minutes in whole-body exposure chamber.
For whole blood collection, we diluted a 5 mM EDTA solution with PBS before beginning collection. A mouse was gently placed in a restrainer and its tail was washed and cleaned using a wipe with 70% alcohol. After grasping the distal end of the tail vein line, we inserted a 1 mL needle, bevel side up, parallel to the skin. Once the needle was placed correctly in the vein, it was slowly removed. Blood was collected from the vein until at least 10 µL was obtained. A 10 µL aliquot of blood was directly mixed with 90 µL of an EDTA solution in a tube and stored at −80 °C.
3. Results and Discussion
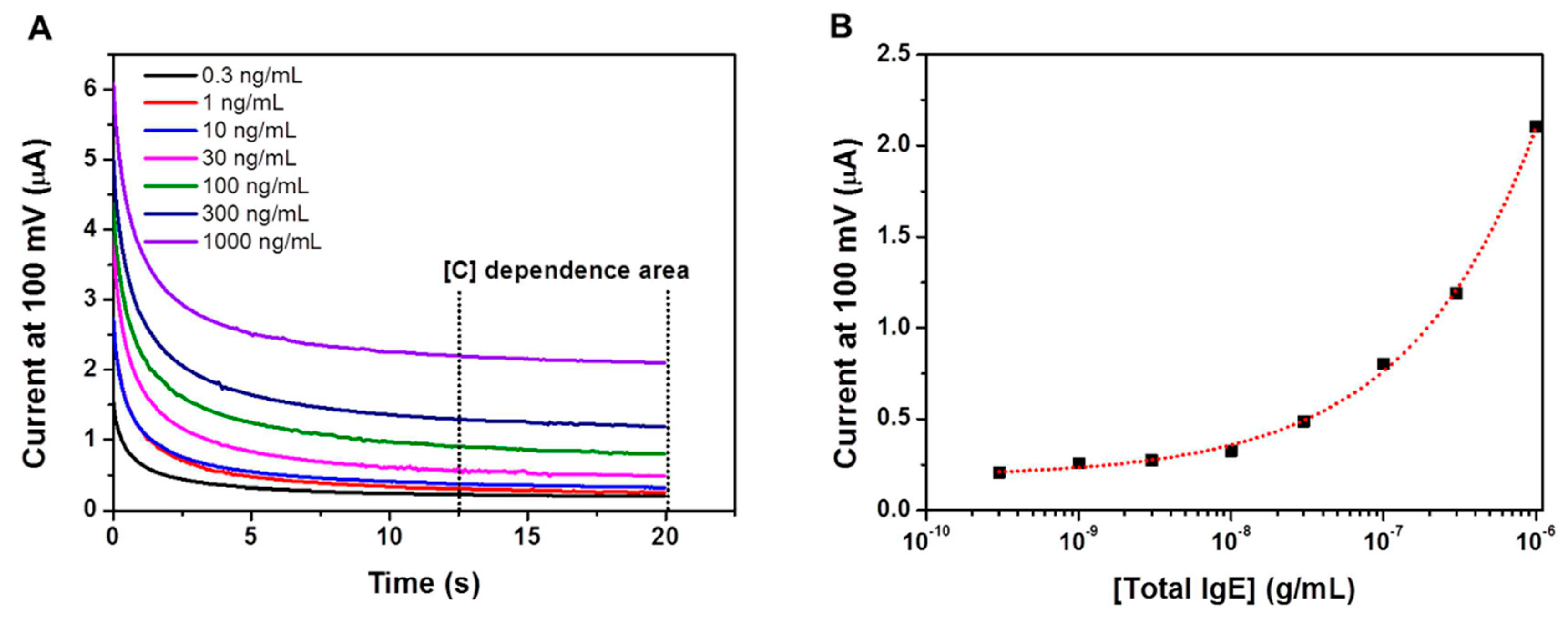
Usually, the conventional ELISA kits have been used TMB as the chromogenic dye which is one of substrates of horseradish peroxidase (HRP). The TMB molecule can be oxidized and reduced by amperometry, and the redox current can be correlated to the OD value [5]. In this work, an immunoassay platform was prepared by immobilizing anti-IgE antibodies to multichannel screen- printed electrode, and IgE in biological sample was bind to anti-IgE antibodies, then HRP conjugated secondary antibodies treated for IgE quantification. TMB was used for the quantification of the amount of bound IgE, and then the amperometric analysis was carried out. As shown in Figure 2A, the amperometric measurements were initially carried out using IgE solutions over the concentration range of 0.3–1000 ng/mL. The amperometric response reached a plateau within 20 s. Absolute current values increased as a function of increasing oxidized-TMB concentration. Figure 2B is the semi-log calibration curve using the current valus obtained at the 20 s of the amperometric measurements.
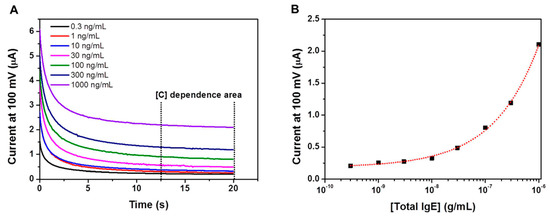
Figure 2.
(A) Amperometry measurement of the EC sensor at 100 mV for different IgE concentrations. (B) EC calibration curve for IgE obtained from IgE solution in mouse blood.
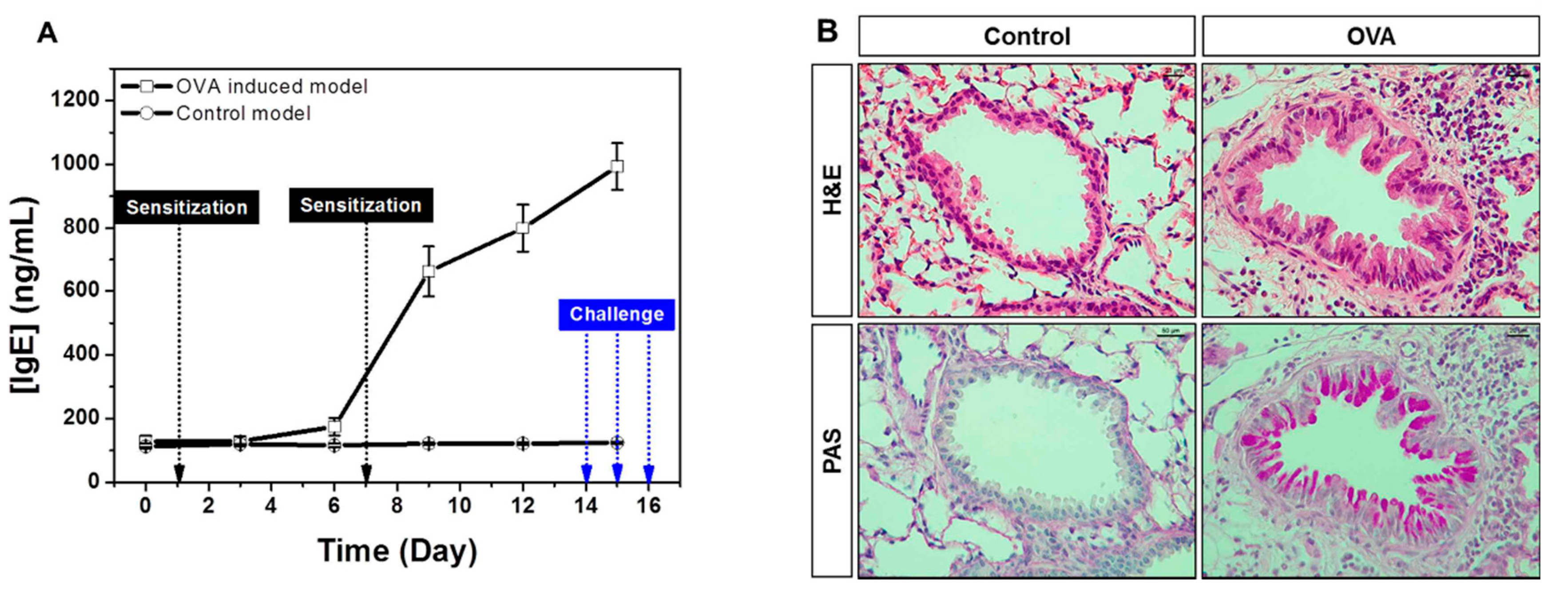
The elevated IgE is the hallmark of type 1 hypersensitivity, which occurs by repeated exposure of allergen. Allergic response initiates in the first allergen sensitization, which is characterized by production of specific IgE driven to the allergen by B cells, and produced IgE binds to high-affinity receptor FcεR1 on the surface of mast cells and basophils, resulting in releases of various mediators including histamine and leukotriene. In challenge phase with same allergen, mast cells and basophils in the airways cause an immediate hypersensitivity through FcεR1. Through this series of processes, IgE-mediated asthma is pathophysiologically characterized by airway inflammation, epithelial hypertrophy, goblet cell hyperplasia and airway hyperresponsiveness (AHR). We performed sequential monitoring of total IgE in whole blood of OVA-induced allergic asthma model. Our results showed that IgE slightly increased after the first OVA challenge and rapidly increased in secondary OVA sensitization as well as during OVA challenge. IgE level further peaked after the last OVA challenge (Figure 3A). In addition, histological analyses showed increased epithelial thickness and mucus production with inflammatory cells infiltration in established asthma model (Figure 3B). Therefore, whole blood IgE measurement using our electrochemical biosensor is a useful tool for sequential monitoring of the onset and progression of the allergic response in the asthma model.
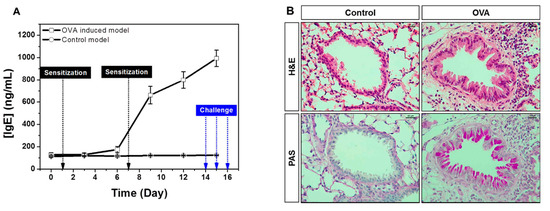
Figure 3.
(A) Sequential monitoring of total IgE with blood (B) histologic changes in OVA-induced mice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aharony, D. Pharmacology of leukotriene receptor antagonists. Am. J. Respire. Crit. Care Med. 1998, 157, S214–S219. [Google Scholar] [CrossRef]
- Pauwels, R.A.; Brusselle, G.J.; Kips, J.C. Cytokine manipulation in animal models of asthma. Am. J. Respire. Crit. Care Med. 1997, 156, S78–S81. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M. Murine models of asthma in understanding immune dysregulation in human asthma. Immunopharmacology 2000, 48, 263–268. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; Salvo, C.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double–walled carbon nanotubes. Anal. Chim. Acta 2017, 959, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, M.; Jose, J.; Kang, M.J.; Pyun, J.C. Electrochemical ELISA based on Escherichia coli with autodisplayed Z-domains. Sens. Actuators B 2012, 175, 46–52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).