Cross-Linked Nanoparticle Membranes for Microelectromechanical Chemical Sensors and Pressure Sensors †
Abstract
:1. Introduction
2. Microelectromechanical Chemical Sensors
2.1. Dynamic Sensing Mode
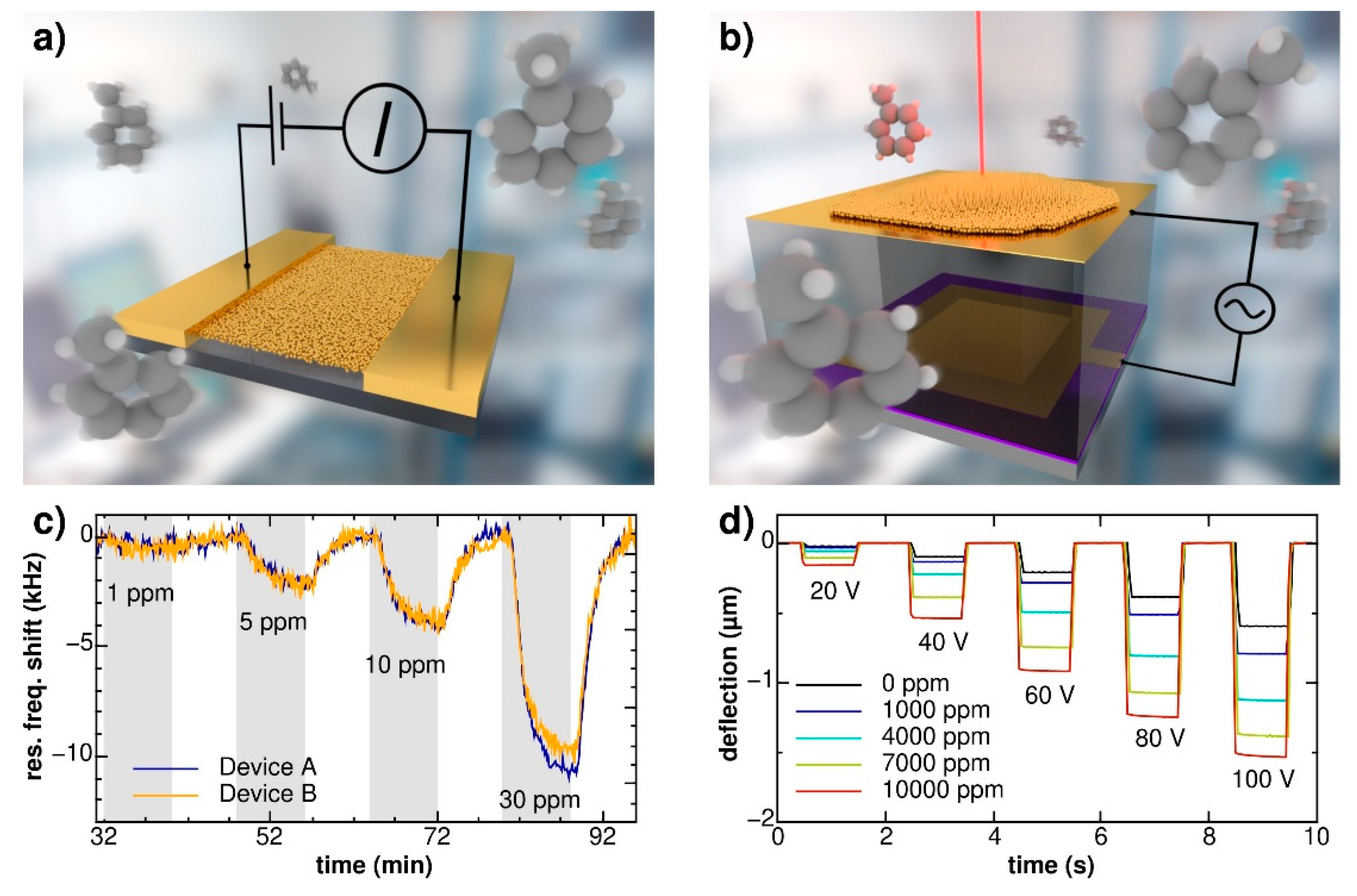
2.2. Quasi-Static Sensing Mode
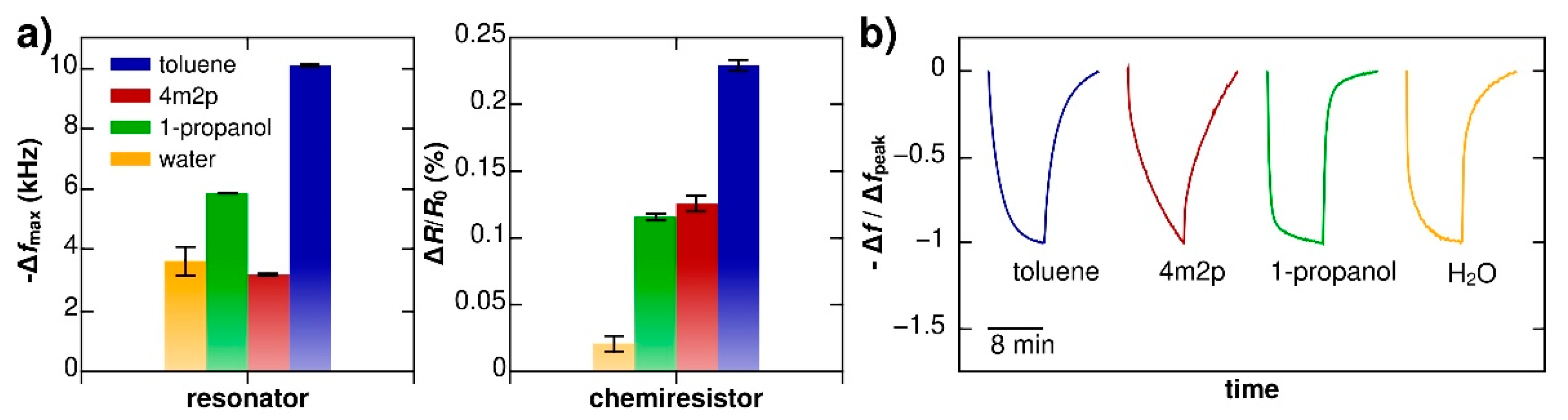
3. Multivariate Sensing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brust, M.; Schiffrin, D.J.; Bethell, D.; Kiely, C.J. Novel gold-dithiol nano-networks with non-metallic electronic properties. Adv. Mater. 1995, 7, 795–797. [Google Scholar] [CrossRef]
- Terrill, R.H.; Postlethwaite, T.A.; Chen, C.-H.; Poon, C.-D.; Terzis, A.; Chen, A.; Hutchison, J.E.; Clark, M.R.; Wignall, G.; Londono, J.D.; et al. Monolayers in Three Dimensions: NMR, SAXS, Thermal, and Electron Hopping Studies of Alkanethiol Stabilized Gold Clusters. J. Am. Chem. Soc. 1995, 117, 12537–12548. [Google Scholar] [CrossRef]
- Herrmann, J.; Müller, K.-H.; Reda, T.; Baxter, G.R.; Raguse, B.; de Groot, G.J.J.B.; Chai, R.; Roberts, M.; Wieczorek, L. Nanoparticle films as sensitive strain gauges. Appl. Phys. Lett. 2007, 91, 183105. [Google Scholar] [CrossRef]
- Vossmeyer, T.; Stolte, C.; Ijeh, M.; Kornowski, A.; Weller, H. Networked Gold-Nanoparticle Coatings on Polyethylene: Charge Transport and Strain Sensitivity. Adv. Funct. Mater. 2008, 189, 1611–1616. [Google Scholar] [CrossRef]
- Schlicke, H.; Rebber, M.; Kunze, S.; Vossmeyer, T. Resistive pressure sensors based on freestanding membranes of gold nanoparticles. Nanoscale 2016, 8, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Wohltjen, H.; Snow, A.W. Colloidal Metal-Insulator-Metal Ensemble Chemiresistor Sensor. Anal. Chem. 1998, 70, 2856–2859. [Google Scholar] [CrossRef]
- Olichwer, N.; Meyer, A.; Yesilmen, M.; Vossmeyer, T. Gold nanoparticle superlattices: Correlating chemiresistive responses with analyte sorption and swelling. J. Mater. Chem. C 2016, 4, 8214–8225. [Google Scholar] [CrossRef]
- Ibanez, F.J.; Zamborini, F.P. Chemiresistive Sensing with Chemically Modified Metal and Alloy Nanoparticles. Small 2012, 8, 174–202. [Google Scholar] [CrossRef] [PubMed]
- Schlicke, H.; Battista, D.; Kunze, S.; Schröter, C.J.; Eich, M.; Vossmeyer, T. Freestanding Membranes of Cross-Linked Gold Nanoparticles: Novel Functional Materials for Electrostatic Actuators. ACS Appl. Mater. Interfaces 2015, 7, 15123–15128. [Google Scholar] [CrossRef] [PubMed]
- Schlicke, H.; Schröter, C.J.; Vossmeyer, T. Electrostatically driven drumhead resonators based on freestanding membranes of cross-linked gold nanoparticles. Nanoscale 2016, 8, 15880–15887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Waitz, R.; Yang, F.; Lutz, C.; Angelova, P.; Gölzhäuser, A.; Scheer, E. Vibrational modes of ultrathin carbon nanomembrane mechanical resonators. Appl. Phys. Lett. 2015, 106, 063107. [Google Scholar] [CrossRef]
- Schlicke, H.; Behrens, M.; Schröter, C.J.; Dahl, G.T.; Hartmann, H.; Vossmeyer, T. Cross-Linked Gold-Nanoparticle Membrane Resonators as Microelectromechanical Vapor Sensors. ACS Sens. 2017, 2, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Schlicke, H.; Bittinger, S.C.; Behrens, M.; Yesilmen, M.; Hartmann, H.; Schröter, C.J.; Dahl, G.T.; Vossmeyer, T. Electrostatically Actuated Membranes of Cross-Linked Gold Nanoparticles: Novel Concepts for Electromechanical Gas Sensors. Proceedings 2017, 1, 301. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlicke, H.; Hartmann, H.; Bittinger, S.C.; Rebber, M.; Behrens, M.; Vossmeyer, T. Cross-Linked Nanoparticle Membranes for Microelectromechanical Chemical Sensors and Pressure Sensors. Proceedings 2018, 2, 821. https://doi.org/10.3390/proceedings2130821
Schlicke H, Hartmann H, Bittinger SC, Rebber M, Behrens M, Vossmeyer T. Cross-Linked Nanoparticle Membranes for Microelectromechanical Chemical Sensors and Pressure Sensors. Proceedings. 2018; 2(13):821. https://doi.org/10.3390/proceedings2130821
Chicago/Turabian StyleSchlicke, Hendrik, Hauke Hartmann, Sophia Caroline Bittinger, Matthias Rebber, Malte Behrens, and Tobias Vossmeyer. 2018. "Cross-Linked Nanoparticle Membranes for Microelectromechanical Chemical Sensors and Pressure Sensors" Proceedings 2, no. 13: 821. https://doi.org/10.3390/proceedings2130821
APA StyleSchlicke, H., Hartmann, H., Bittinger, S. C., Rebber, M., Behrens, M., & Vossmeyer, T. (2018). Cross-Linked Nanoparticle Membranes for Microelectromechanical Chemical Sensors and Pressure Sensors. Proceedings, 2(13), 821. https://doi.org/10.3390/proceedings2130821




