1. Introduction
The detection of toxic gas hydrogen sulfide (H2S), which is well known for its characteristic stench of rotten eggs, has a major importance for the oil and gas industry as well as other industries like wastewater treatment plants. These chemical industries produce H2S gas continually, although it is extremely hazardous for living beings. Even at low gas concentrations and short exposure time, H2S can cause substantial physical damage. Its toxicity is primarily due to the inhibition of metal-containing enzymes through sulfide formation or through the reduction of disulfide bridges [
1]. For this reason, the particularly affected areas are the mucous membranes and tissues with a high oxygen demand like nerves and the heart [
1]. Moreover, at higher gas concentrations H2S has the treacherous property to stun the olfactory receptors, what makes it even more dangerous. All of this shows that the effect of H2S should be taken seriously and that there is a mandatory need for its monitoring with regard to safety in the workplace. Furthermore, it is quite corrosive and damages especially metallic facilities. One of the most commonly used detection method is currently based on electrochemical sensors. This technology offers a good selectivity to H2S, but also a limited lifetime [
2], which makes it quite expensive.
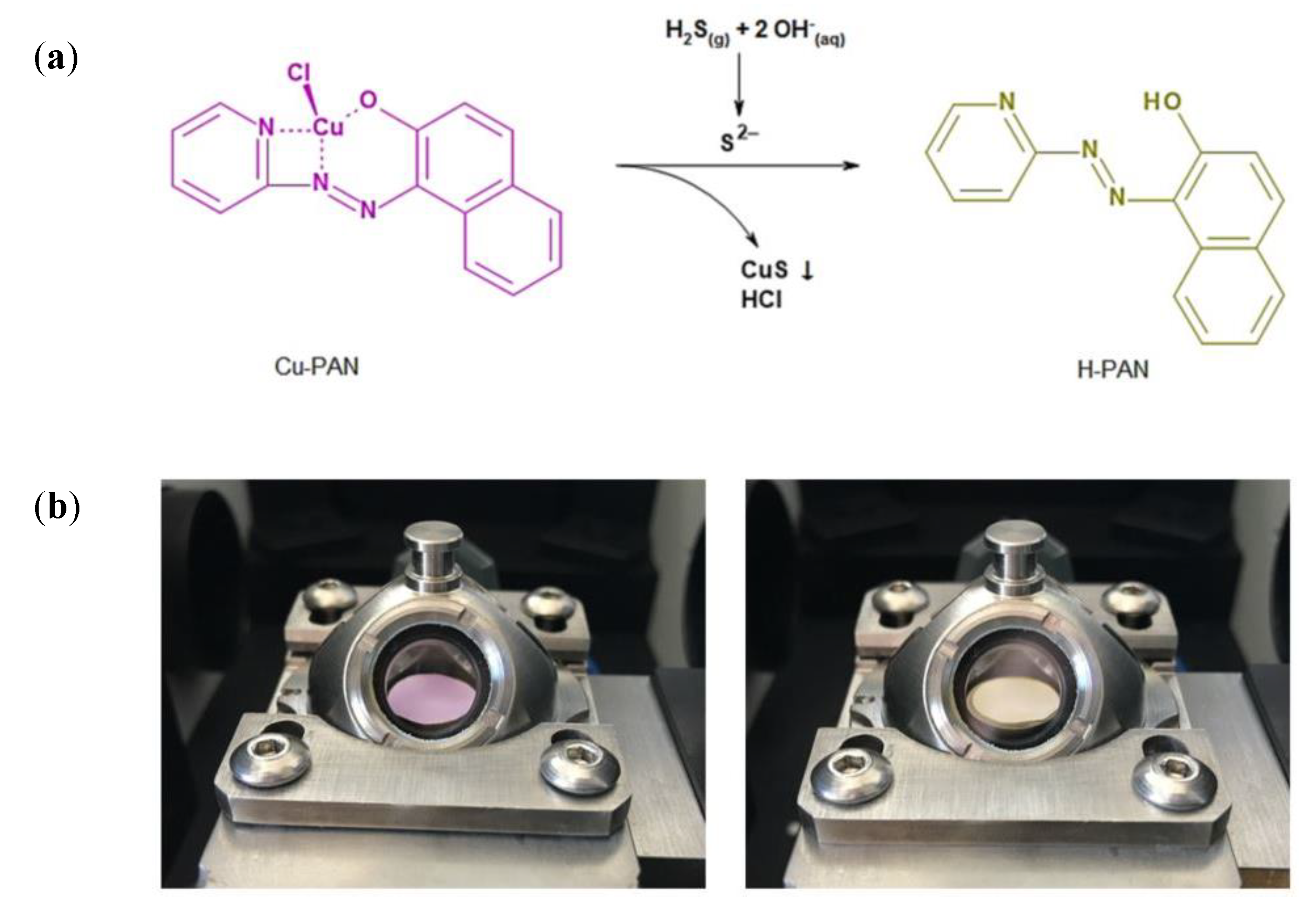
We describe a highly sensitive and fast method for the detection of H2S based on a gasochromic reaction. Our method is based on a printed test strip, which is as simple to use as a pH-indicator strip for example. As color changing material Cu-PAN, a copper(II) complex of the azo dye 1-(2-pyridylazo)-2-naphtol (H-PAN) is used, which changes its color from purple to yellow, if H2S is present. In this abstract, the measurement results of embedded Cu-PAN screen printed on different substrates are shown.
2. Materials and Methods
2.1. Preparation of the Cu-PAN Test Stripes
The Cu-PAN complex was prepared as described in literature [
3,
4]. A solution of 0.5 mmol copper(II) chloride dihydrate (≥99.99% trace metals basis, ALDRICH Chemistry) in 3 mL distilled water was added to a solution of 0.5 mmol 1-(2-pyridylazo)-2-naphtol (Indicator Grade, SIGMA-ALDRICH) in 75 mL ethanol (HPLC Gradient Grade, ROTH). Stirring the solution at room temperature overnight resulted in a black/dark red powder, which was subsequent filtered and washed three times with 3 mL ethanol and dried at room temperature.
To yield a paste suitable for screen printing, the copper(II) complex was first finely crushed and then embedded in a plasticized ethyl cellulose matrix using ethanol as solvent. All prints were performed on a Thieme LAB 1000 (THIEME GmbH & Co. KG, Teningen, Germany) screen printing machine using a 180-27 polyester screen for low level of paste application. A transparent PE foil (Autostat CT3, Gauge 75 µm, MacDermid Autotype Ltd., Wantage, UK) and a high-quality offset paper (UPM Finesse Premium Silk, 150 g/m2, UPM Communication Paper, Augsburg, Germany) were used as substrates. All samples were dried at room temperature for 12 h. The layer thickness of the twofold wet-in-wet prints were determined on the PE foil to 0.9–1.1 µm by 3D laser scanning microscopy (KEYENCE VK 9700 K, Keyence Deutschland GmbH, Neu-Isenburg, Germany).
2.2. H2S Gas Measurements
To assess the quality of the printed indicator layers, gas measurements were performed at the gas measurement station of Fraunhofer IPM. The general description of the gas measurement station is given in [
5]. The color change was measured by UV/VIS spectroscopy (Perkin-Elmer Lambda900, Perkin-Elmer, Waltham, MA, USA) using a diffuse reflection accessory (Praying Mantis, Harrick Scientific Products Inc., Waltham, MA, USA). This measurement setup allows determining the diffuse reflectance of solids with a high surface roughness as well as powder samples. To adjust the size of the prints to the measurement setup, circles with a diameter of 10 mm were cut out of the printed indicator stripes. For the measurement of the transparent PE foil, a blank paper underneath the probe served as support. The gas measurements were performed in synthetic air and a relative humidity of 40% at room temperature.
3. Results and Discussion
Figure 1b shows the gasochromic reaction of the printed Cu-PAN layer from purple to yellow on a paper substrate after the injection of 20 ppm H2S into the gas measurement chamber of the diffuse reflection assembly. This color change is also easily to recognize with the naked eye.
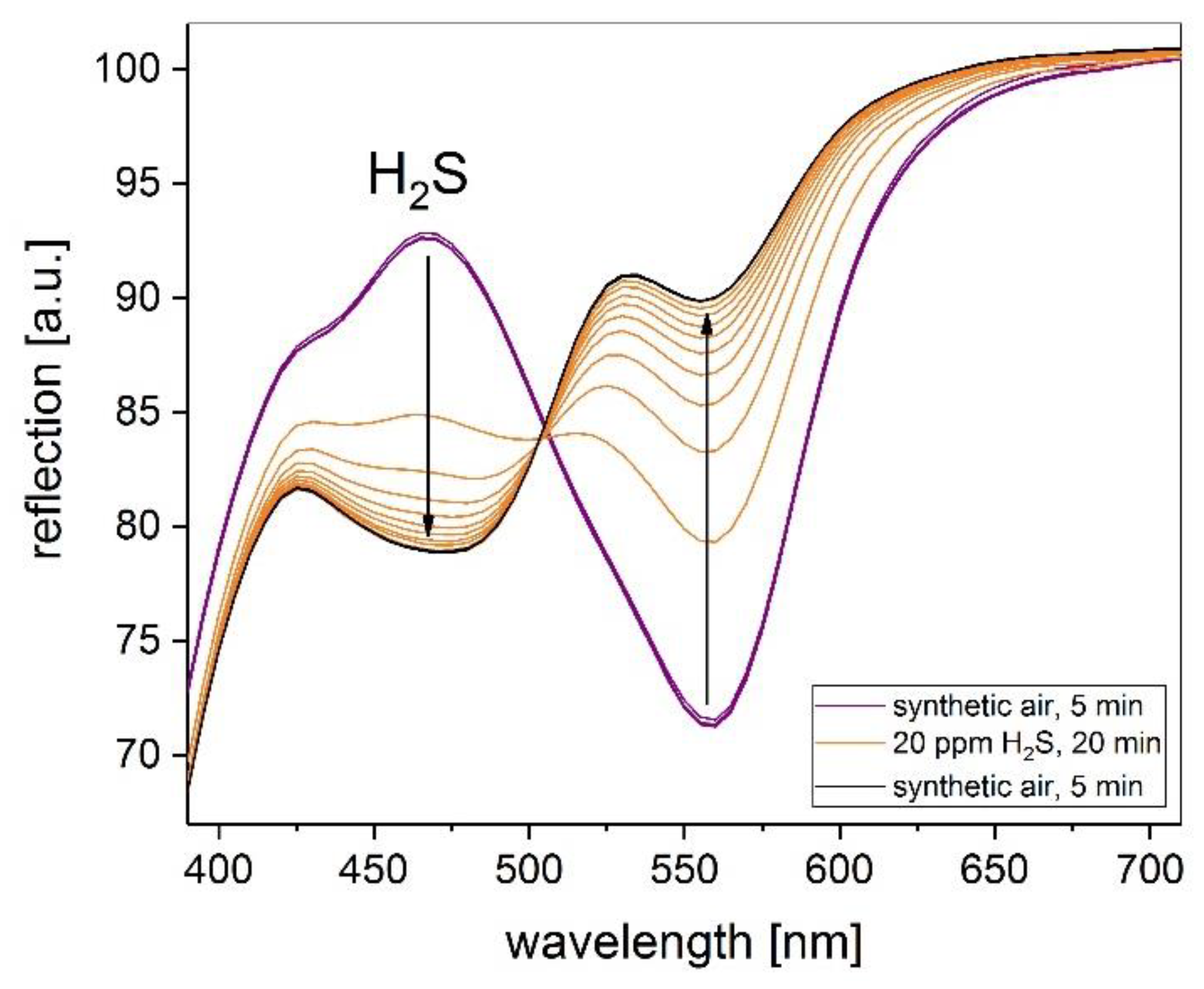
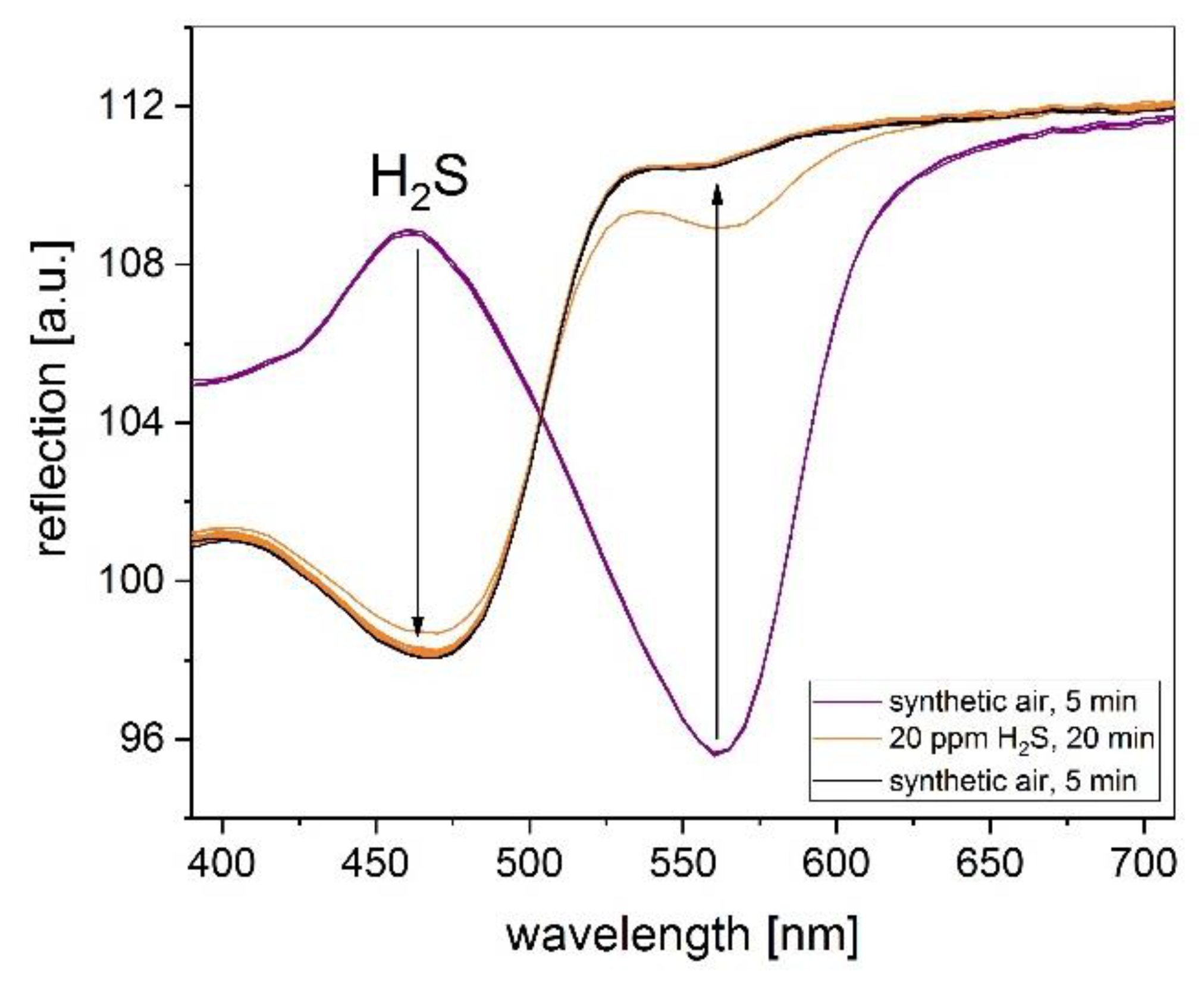
Figure 2 and
Figure 3 show the related reflection spectra before and after the exposure to 20 ppm H2S of the Cu-PAN complex printed on coated paper and PE foil, respectively. After reaction with H2S a clear color change from purple to yellow can be detected for all prepared layers. The main change in reflection is located in the wavelength range of 460 nm (blue) and 560 nm (yellow). The comparison between the substrates shows a significant faster reaction for using PE foil as substrate. In less than one minute of H2S exposure, the color of the layer changes from purple to yellow. However, the reflection change at 560 nm due to H2S exposure is much higher for the coated paper.
At lower gas concentrations, the color change is slowed down for both substrates, but even 1 ppm H2S is easy to detect. Repetition of the gas measurements after the storage of the samples in an unsealed plastic cover at room temperature for one month provided a reproducible delta of reflection.
Analysis of STA (Simultaneous Thermal Analysis) measurement data (NETZSCH STA 409 C/CD, Netzsch, Germany) exhibit a high thermal stability (~90 °C) of the Cu-PAN complex. Under these aspects and due to the fact, that this Cu-PAN complex has a slow back reaction (complete back reaction within days), it seems to be a promising material for a fast single-use H2S indicator. The color change could be detected by a photo camera or even through human eye, easily. That offers the opportunity to develop a low-cost fast H2S sensitive sensor based on Cu-PAN with no need of complex and expensive components or electronics.
4. Conclusions
Our investigations illustrate the properties of a printed gasochromic Cu-PAN complex layer to monitor H2S in ambient air on basis of a color change from purple to yellow. The speed of the color change is dispositive influenced through the choice of the substrate. For the manufacturing of the indicator strip, a paste was developed which is suitable for screen printing. With regard to the easy and inexpensive preparation, the usage of the Cu-PAN complex could be conceivable in many environmental, biological and industrial applications.
Author Contributions
L.E. and J.W. conceptualization, L.E., C.P. and K.R.T. conceived and designed the experiments; L.E. performed the experiments; L.E., K.R.T. and C.P. analyzed the data; L.E. wrote the paper.
Funding
This research was funded by a Eurostars program and result from collaboration with Sensotran, s. I., Scemtec Transponder Technology GmbH and the University of Barcelona.
Acknowledgments
We like to thank THIEME GmbH & Co. KG in Teningen for the realization of the prints.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwefelwasserstoff. The MAK-Collection for Occupational Health and Safety: American Cancer Society. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/3527600418.mb778306d0043 (accessed on 6 July 2018).
- Datasheet Electrochemical DrägerSensor. Available online: https://www.draeger.com/Products/Content/sensor-ec-pi-9045596-en-master.pdf (accessed on 26 April 2018).
- Carpenter, T.S.; Rosolina, S.M.; Xue, Z.-L. Quantitative, colorimetric paper probe for hydrogen sulfide gas. Sens. Actuators B Chem. 2017, 253, 843–851. [Google Scholar] [CrossRef]
- Ariza-Avidad, M.; Agudo-Acemel, M.; Salinas-Castillo, A.; Capitán-Vallvey, L.F. Inkjet-printed disposable metal complexing indicator-displacement assay for sulphide determination in water. Spectrochimica 2014, 872, 55–62. [Google Scholar] [CrossRef]
- Kneer, J.; Eberhardt, A.; Walden, P.; Ortiz Pérez, A.; Wöllenstein, J.; Palzer, S. Apparatus to characterize gas sensor response under real-world conditions in the lab. Rev. Sci. Instrum. 2014, 85, 055006. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).