A Microfabricated 4-Electrode Conductivity Sensor with Enhanced Range †
Abstract
:1. Introduction
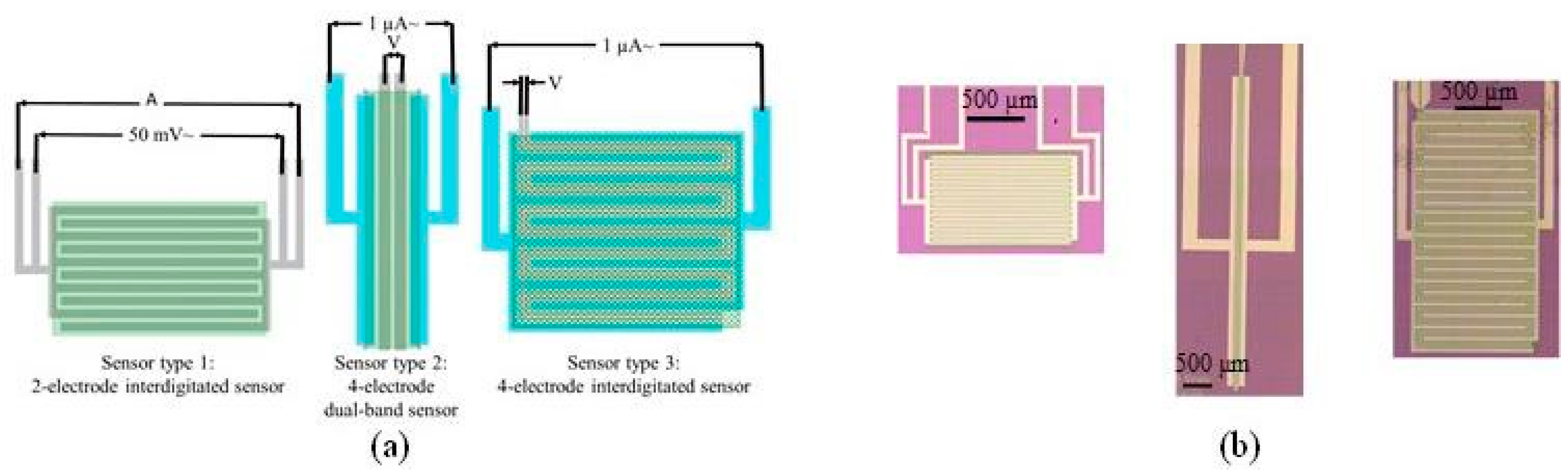
2. Design
2.1. 2-Electrode Sensors
2.2. 4-Electrode Sensors
3. Experimental
3.1. Device Fabrication
3.2. Measurement Setup
4. Results
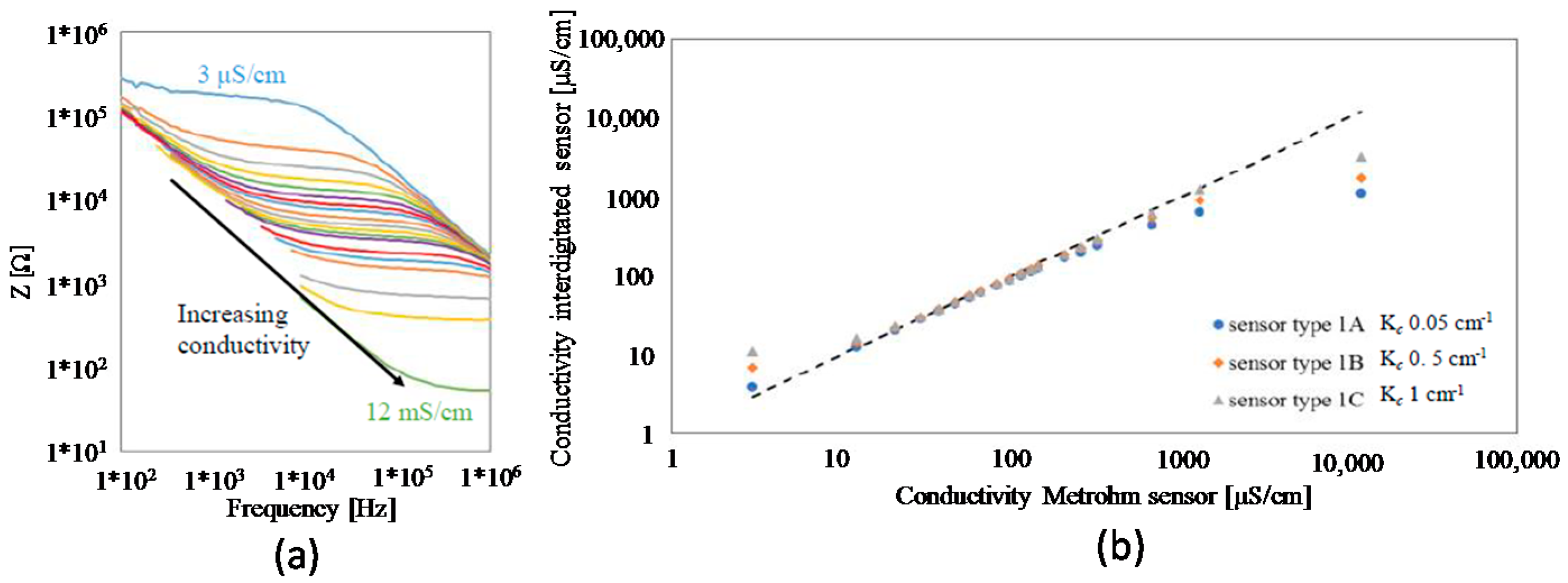
4.1. 2-Electrode Sensor
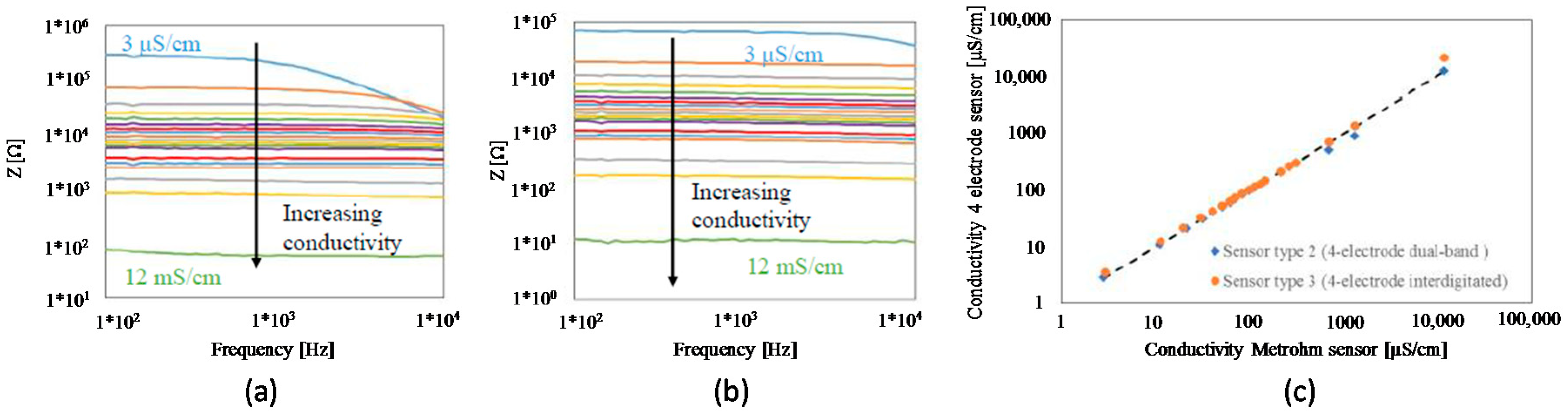
4.2. 4-Electrode Sensor
5. Conclusions
References
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Langereis, G. An Integrated Sensor System for Monitoring Washing Processes. Ph.D. Thesis, University of Twente, Twente, The Netherlands, 1999. [Google Scholar]
- Olthuis, W.; Streekstra, W.; Bergveld, P. Theoretical and experimental determination of cell constants of planar-interdigitated electrolyte conductivity sensors. Sens. Actuators B 1995, 24–25, 252–256. [Google Scholar] [CrossRef]
- Timmer, B.; Sparreboom, W.; Olthuis, W.; Bergveld, P.; van den Berg, A. Optimization of an electrolyte conductivity detector for measuring low ion concentrations. Lab Chip 2002, 2, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Schroder, D.K. Semiconductor Material and Device Characterization, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–59. [Google Scholar]
| Sensor Type | Sa [µm] | Size Measurement (Inner) Electrode | Size Outer Electrode | ||||
|---|---|---|---|---|---|---|---|
| Wb [µm] | Lc [µm] | Nd | Wb [µm] | Lc [µm] | Nd | ||
| Type 1 A,B,C | 10 | 20 | 1220 | A: 250, B: 26, C: 13 | |||
| Type 2 | 10 | 20 | 5300 | 2 | 80 | 5300 | 2 |
| Type 3 | 10 | 20 | 26,860 | 2 | 50 | 1160 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brom-Verheijden, G.J.A.M.; Goedbloed, M.H.; Zevenbergen, M.A.G. A Microfabricated 4-Electrode Conductivity Sensor with Enhanced Range. Proceedings 2018, 2, 797. https://doi.org/10.3390/proceedings2130797
Brom-Verheijden GJAM, Goedbloed MH, Zevenbergen MAG. A Microfabricated 4-Electrode Conductivity Sensor with Enhanced Range. Proceedings. 2018; 2(13):797. https://doi.org/10.3390/proceedings2130797
Chicago/Turabian StyleBrom-Verheijden, Greja J. A. M., Martijn H. Goedbloed, and Marcel A. G. Zevenbergen. 2018. "A Microfabricated 4-Electrode Conductivity Sensor with Enhanced Range" Proceedings 2, no. 13: 797. https://doi.org/10.3390/proceedings2130797
APA StyleBrom-Verheijden, G. J. A. M., Goedbloed, M. H., & Zevenbergen, M. A. G. (2018). A Microfabricated 4-Electrode Conductivity Sensor with Enhanced Range. Proceedings, 2(13), 797. https://doi.org/10.3390/proceedings2130797




