Abstract
We demonstrated the possibility of selective detection of hydrogen, ethanol, hydrogen sulfide, carbon monoxide, methane and ammonia gas using metal oxide gas sensor operating in non-stationary thermal regime. This non-stationary regime consists in fast heating of the sensing layer to high temperature followed by temperature stabilization at lower temperature. The analysis of the shape of the curve describing the sensor resistance as a function of time during this measurement cycle enables the quantitative analysis of gas mixture. The investigation of kinetics of the process in non-stationary regime permits to understand the mechanism of the processes on the surface of sensing material.
1. Introduction
During last 15–20 years, the main direction of the development of sensor science was a material science, the development and improvement of sensing materials. The researchers looked for new substances, materials, which could be used for the fabrication of sensing elements. As well, the scientists improved the synthetic procedures used for the preparation of these materials. Unfortunately, the progress, first, from the point of view of improvement of sensor selectivity, in comparison with traditional sensing nano-materials is not very strong. However, there is a practically important problem of selective detection of gases, and this problem must be solved.
On the other hand, chemometric analysis of the multidimensional data, obtained in multisensor instrument enables, in principle, the solution of the problem of qualitative chemical analysis. However, the application of “electronic nose” implies important restrictions. An increase in the number of gas sensors in the array leads to an increase in the instability of such a system as a whole with the rate of geometric progression. In contrast, a decrease in the number of sensors not only leads to an improvements of system stability, but also decreases power consumption, which is very important in autonomous instruments.
The most reasonable approach to the problem of qualitative gas analysis, according to our opinion, is the application of non-stationary regimes of metal oxide sensor operation, in particular, the application of temperature modulation of the sensor. In the later case, the volume of information about gas medium increases because the processes of chemisorption of reducing gas, processes of its interaction with chemisorbed oxygen, and desorption of reaction products from the surface are partially separated in time. Because of this, there is a possibility to reveal individual characteristics of gases to be analyzed.
It is clear that the application of temperature modulation of metal oxide gas sensor does not lead immediately to the realization of the procedure of qualitative gas analysis. If sensing layer resistance as a function of time has the same shape in each measurement cycle, it is impossible to distinguish one gas from another. It is necessary to use gas sensing layers, which could give in combination with specially selected temperature regimes typical extrema of functions characterizing sensor resistance as a function of time during measurement cycle. These extrema can make easier the problem of qualitative analysis, permit us to distinguish one gas from another in a wide range of concentrations. Nevertheless, the fixation of appropriate regimes of sensor operation is rather complicated task requiring precise experiments.
Instead of smooth sinusoidal regime of sensor temperature modulation used by a number of researchers, we suggest to use fast impulse heating and relatively fast cooling down. The application of impulse heating regime enables the saving of heating power and, respectively use autonomous power source. In addition, this approach permits an increase in efficiency of qualitative gas analysis. Optimal combination of the composition of gas sensing layer and regime of temperature modulation is necessary but not sufficient condition for the solution of the problem of qualitative gas analysis. It is necessary also to carry out a correct chemometric analysis of the arrays of multidimensional data. One of aims of this work was the formalization of the procedure of the solution of the problem of qualitative analysis of gas. This procedure was applied using the analysis of data from single sensor. However, this procedure can be extended to the treatment of data given by multisensor array (“electronic nose”).
In our earlier work [1], we tested the impulse temperature regimes for gas sensing layers of different composition. However, results applicable for the qualitative analysis of such gas as hydrogen were obtained only using “classical” sensing material – tin dioxide decorated with palladium or decorated with palladium and platinum. Thus, the choice of the composition of gas sensing layer was dictated by concrete task, qualitative analysis of hydrogen in air.
2. Experiment
For the fabrication of gas sensing layer of the sensors, we used tin dioxide nanopowder obtained from stannic acid. For this, we added ammonia solution to the solution of tin acetate (+4) in glacial acetic acid. Stannic acid was separated from the solution in centrifuge, washed by deinized water, dried and calcinated. As a result, we obtained nano-powder of tin dioxide.
As a dopant, we used a solution of tetraamminopalladium nitrate (+2). To get printable ink, the powder consisting of a mixture of tin dioxide powder with dopant was mixed in a mortar with viscous vehicle, solution of ethylcellulose in terpineol. This ink was deposited as a thick (~10 micron thick) layer onto a substrate containing platinum electrodes and platinum microheater. The size of the substrate was of 2.5 × 0.5 mm. After sensing layer deposition, the substrate was dried at 423 K and annealed at 1023 K. At this temperature, fragile gel with high specific area (~100 m2/g) was formed. Palladium from the complex compound was reduced at this temperature, final palladium content in the sensing material was of 3 wt. %. After formation of the sensing layer, the substrate was packaged into TO-8 package.
The study of gas response of the sensor was carried out at constant gas flow equal to 200 cm3/min. We used standard etalon gas mixture, the gas was diluted with synthetic air. Constant relative gas humidity equal to 33% was reached by bubbling of a part of gas flow through distilled water, this part of gas flow was saturated with water to the humidity close to 100%. The flow rates were controlled using Bronkhorst F-111BI-1k0 mass flow controllers.
Temperature of the sensing element was controlled by electronic unit, platinum microheater of the sensor was used as thermometer. In temperature modulation regime, the heating cycle duration was of 15 s, first 2 s of this period corresponded to the heating up to 723 K, whereas final 13 s corresponded to the cooling and stabilization to 373 K. The choice of heating and cooling time was due to the internal thermal time constant of the sensor chip equal to about 1 s. The sensor response was calculated as a ratio of the sensor resistance in pure air R0 to the resistance in gas medium under investigation R.
3. Results and Discussion
The shape of the curves (sensor signal as a function of time) can characterize both the nature of the gas and its concentration. This analysis is possible, because non-stationary thermal mode allows discovery of individual peculiarities of each gas caused by its sorption on the surface of the gas sensing layer, chemical interactions and product desorption. It is necessary to not that CH4 and NH3 demonstrate their individual properties relatively slightly (Figure 1). This is related, probably, with their chemical stability in temperature range chosen for the experiments. The curves characterizing the sensor resistance as a function of time have a typical maximum in the beginning of cooling period, marked by arrow in Figure 2. This maximum is observed only for the sensors decorated with Pd and only for H2 and gases easily dehydrogenized on catalyst at temperature below 450 °C:
CH3 − CH2 − OH ↔ CH3 − CHO +H2
H2S ↔ H2 + S
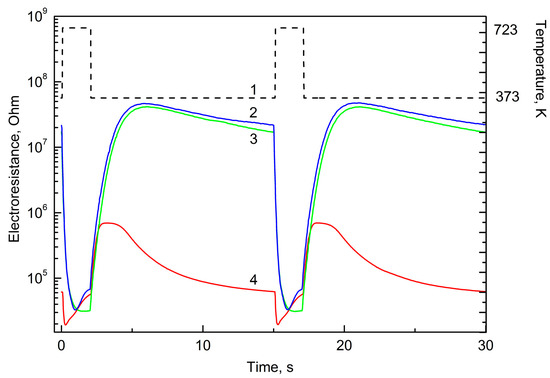
Figure 1.
Time-resistance dependence for temperature (1), 100 ppm ammonia (2), 100 ppm methane (3), 100 ppm carbon monoxide (4) during two measurement periods.
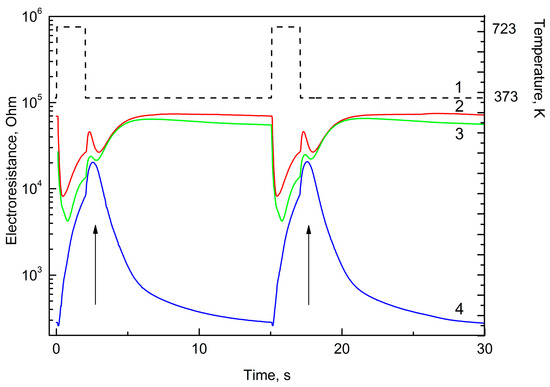
Figure 2.
Time-resistance dependence for temperature (1), 100 ppm hydrogen (2), 100 ppm ethanol (3), 50 ppm hydrogen sulfide (4) during two measurement periods.
This maximum could be explained by the following. In the beginning of the measurement cycle, that is at 100 °C, on the surface there is high concentration of adsorbed analyte (reducing gas) not able to be oxidized at this temperature (adsorption increases ant low temperature). At fast heating, at higher temperature hydrogen can be oxidized:
H2 → 2H+
However, the formation of large concentration of hydrogen cations on the surface leads to fast interaction with excess of oxygen anions and to the formation of water:
2H+ + O2− → H2O
This, in turn, leads to a decrease in hydrogen concentration on the surface and to increase in resistance. Results of H2/CH4 discrimination using PC (principle component) analysis is presented in Figure 3, demonstrating clear monotone curve.
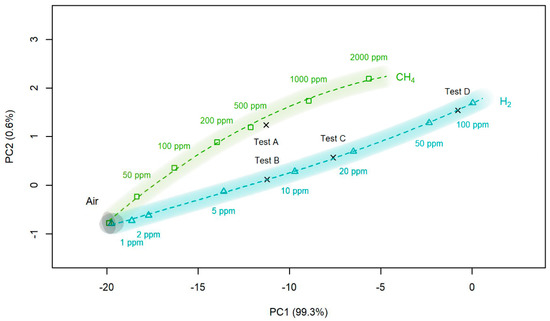
Figure 3.
Principal component analysis of hydrogen and methane. The picture demonstrating monotone dependence of PC point position on gas concentration. In this way, it is possible to determine not only kind of gas, but also its concentration.
4. Conclusions
The application of Pulsing heating mode of semiconductor gas sensor operation consisting in sharp heating and cooling of the gas sensor in combination with principle component analysis of the curve enables selective qualitative determination of the composition of admixture to air using single gas sensor. The most important advantage of this analysis compared to traditional “electronic nose” technology consists in much better stability of the results and in the possibility to determine in this way not only qualitative composition of gas mixture, but also to measure concentration of gases using PC curve.
Author Contributions
A.S. performed main part of experiments, P.M. was responsible for data treatment and development of mathematical models, A.V. provided gas sensors and participated in experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Reference
- Shaposhnik, A.; Zviagin, A.; Sizask, E.; Ryabtsev, S.; Vasiliev, A.; Shaposhnik, D. Acetone and ethanol selective detection by a single MOX-sensor. Procedia Eng. 2014, 87, 1051–1054. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).