Fast Enthalpy-Sensing Microsystem Operating in Continuous Flow †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prerequisite for Calibration: Suitable Fluids and Ways to Handle Their Mixing
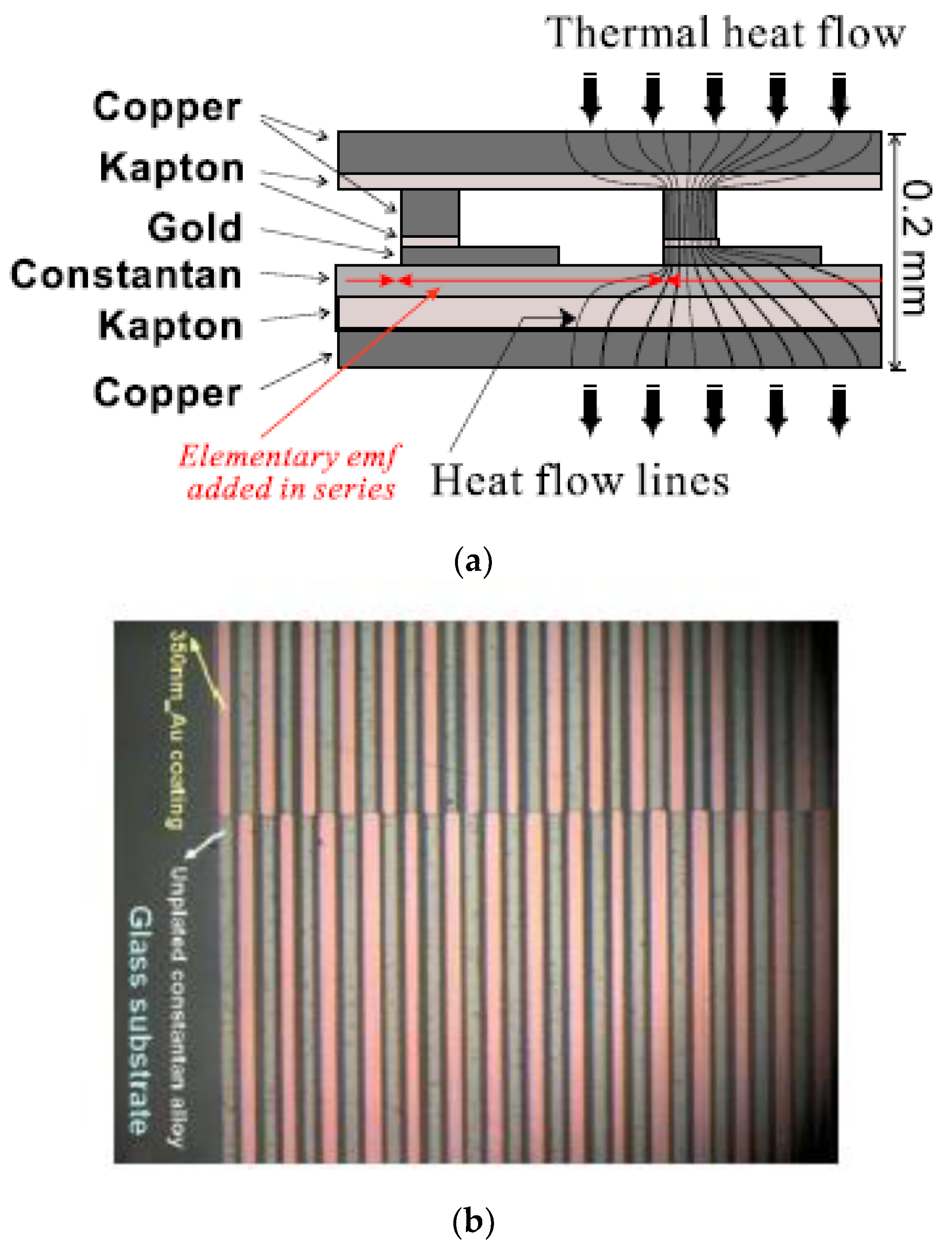
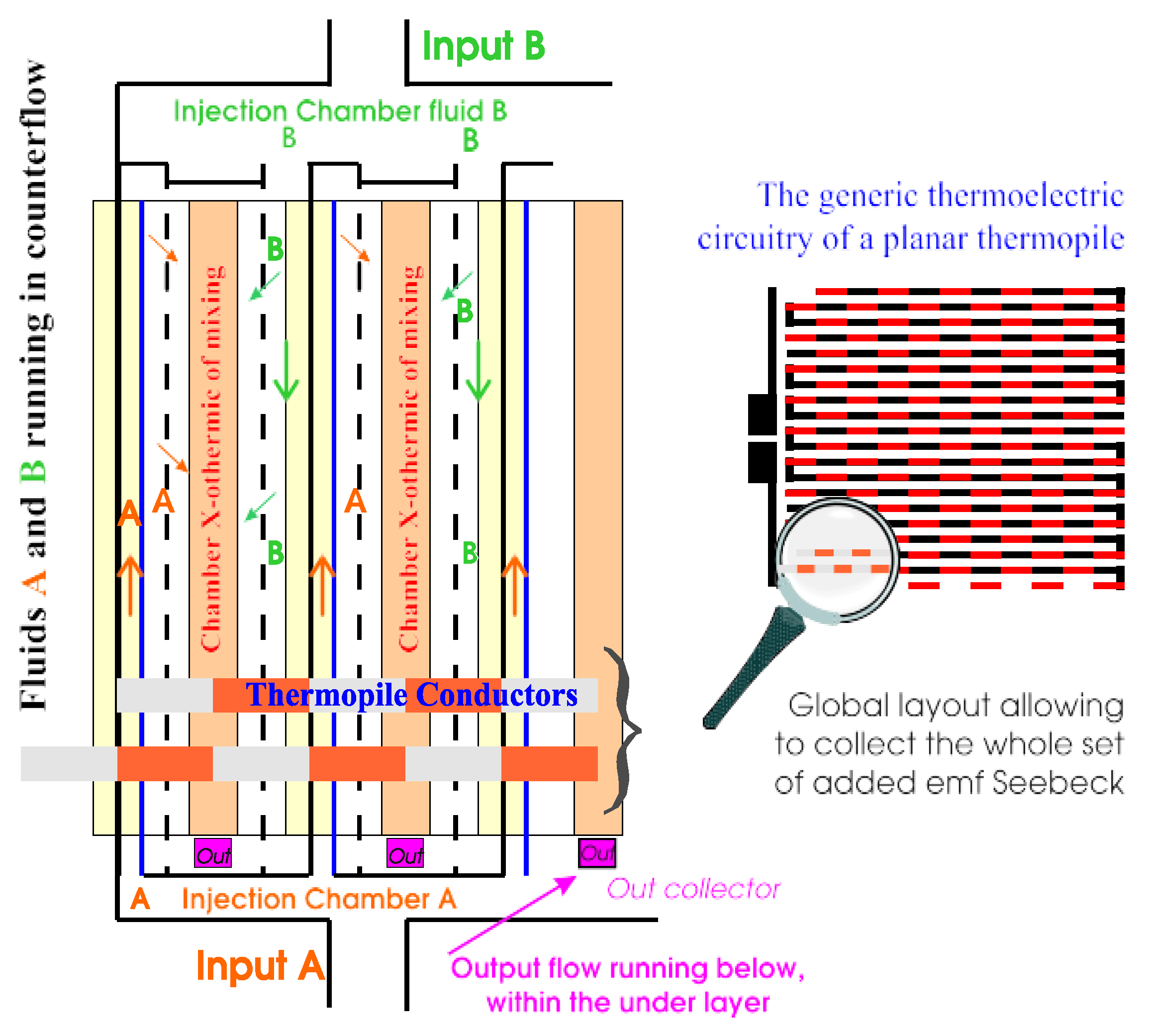
2.2. Generic Design for Monitoring Excess Enthalpies
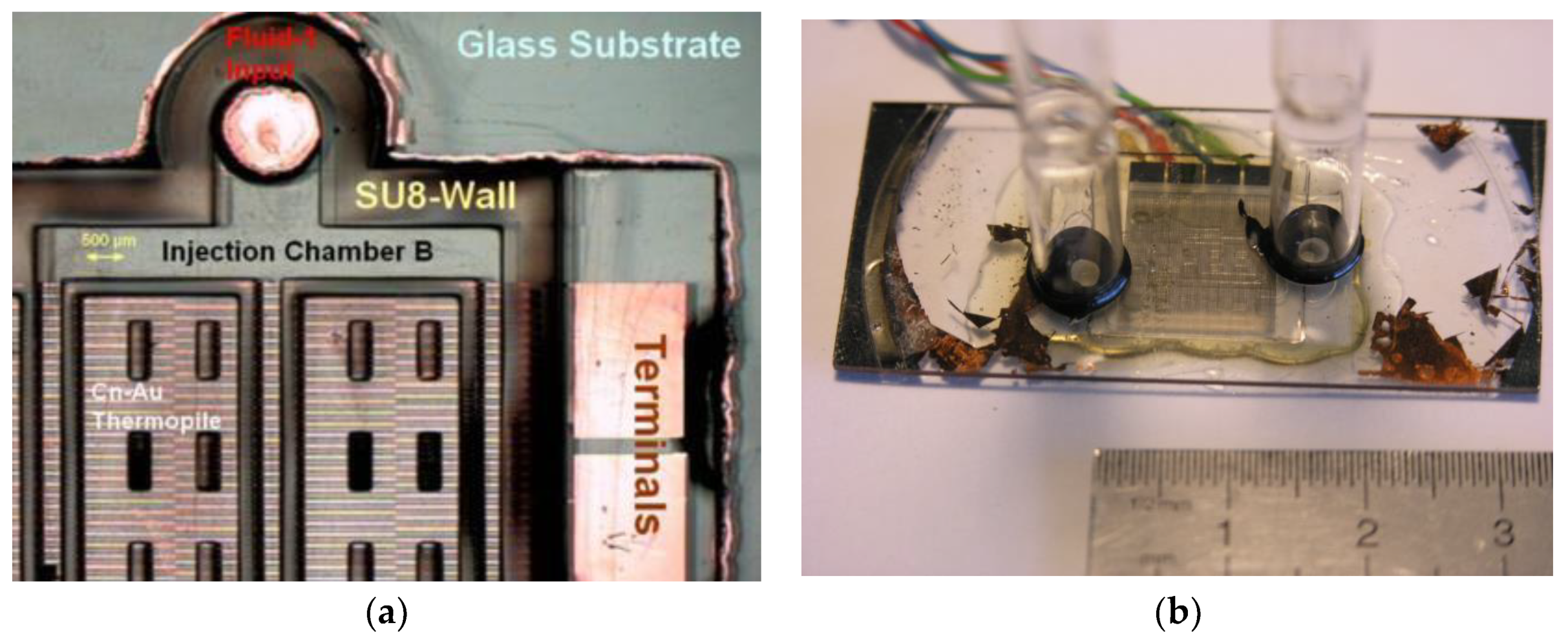
2.3. Specific Design with Micro Technology
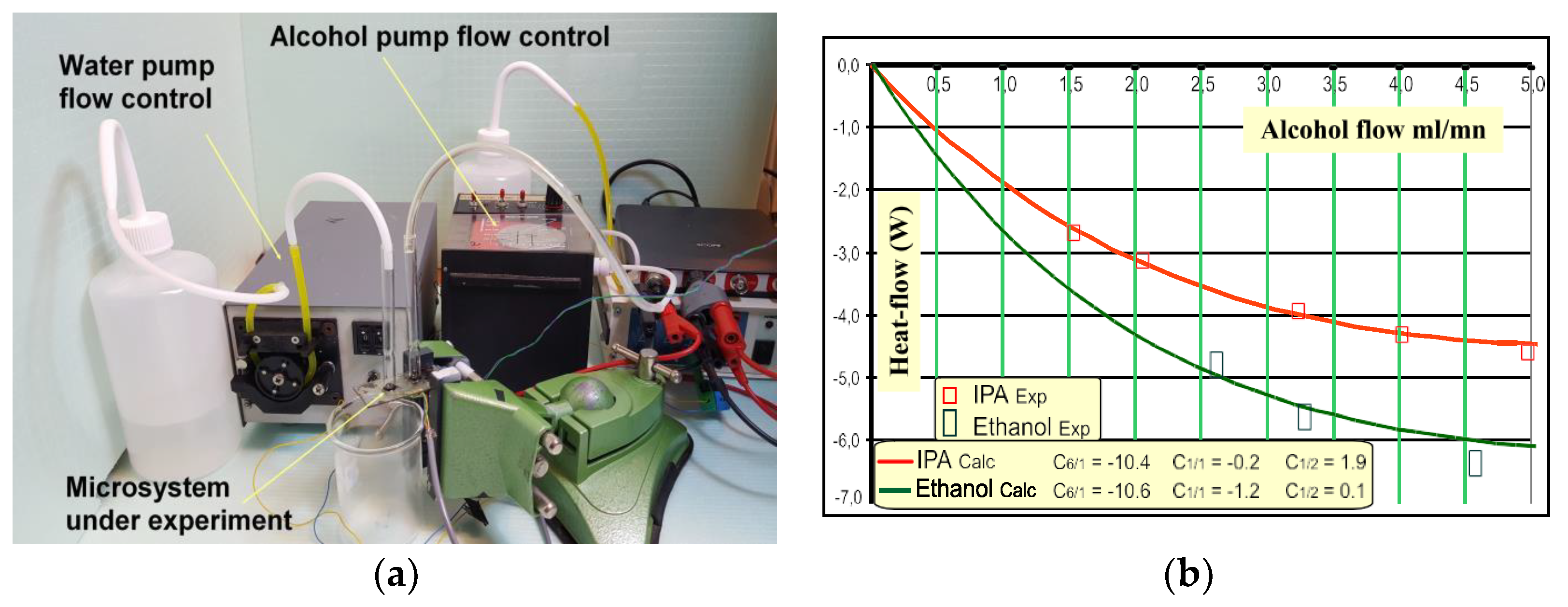
3. Results and Brief Discussion: Technological Achievement and Example of Output Signals
References
- Bejan, A. Entropy Generation Through Heat and Fluid Flow; Wiley & Sons: Hoboken, NJ, USA, 1960. [Google Scholar]
- Shirtliffe, C.J.; Tye, R.P. Guarded Hot Plate and Heat Flow Meter Methodology; ASTM Special Technical Publication: Philadelphia, PA, USA, 1985. [Google Scholar]
- Gaviot, E.; Failleau, G.; Camberlein, L.; Polet, F.; Morice, R.; Bêche, B. Metrological prospects for the assessment of transition plateaus. Metrologia 2010, 47, 349–356. [Google Scholar] [CrossRef]
- Gaviot, E.; Failleau, G.; Camberlein, L.; Polet, F.; Morice, R.; Bêche, B. Towards a thermodynamic assessment of transition plateaus. Metrologia 2010, 47, 357–362. [Google Scholar] [CrossRef]
- Giordani, N.; Camberlein, L.; Gaviot, E.; Polet, F.; Bêche, B. Design and experimental validation of SU-8 based Micro-psychrometers. IEEE Trans. Instrum. Meas. Technol. 2007, 56, 102–106. [Google Scholar] [CrossRef]
- Boyne, J.A.; Williamson, A.G. Enthalpies of mixing of Ethanol and Water at 25 °C. J. Chem. Eng. Data 1967, 12, 318. [Google Scholar] [CrossRef]
- Peeters, D.; Huyskens, P. Endothermicity or exothermicity of water/alcohol mixtures. J. Mol. Struct. 1993, 300, 539–550. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhammedi, T.; Camberlein, L.; Polet, F.; Bêche, B.; Gaviot, E. Fast Enthalpy-Sensing Microsystem Operating in Continuous Flow. Proceedings 2018, 2, 771. https://doi.org/10.3390/proceedings2130771
Mhammedi T, Camberlein L, Polet F, Bêche B, Gaviot E. Fast Enthalpy-Sensing Microsystem Operating in Continuous Flow. Proceedings. 2018; 2(13):771. https://doi.org/10.3390/proceedings2130771
Chicago/Turabian StyleMhammedi, Taoufik, Lionel Camberlein, Frédéric Polet, Bruno Bêche, and Etienne Gaviot. 2018. "Fast Enthalpy-Sensing Microsystem Operating in Continuous Flow" Proceedings 2, no. 13: 771. https://doi.org/10.3390/proceedings2130771
APA StyleMhammedi, T., Camberlein, L., Polet, F., Bêche, B., & Gaviot, E. (2018). Fast Enthalpy-Sensing Microsystem Operating in Continuous Flow. Proceedings, 2(13), 771. https://doi.org/10.3390/proceedings2130771




