Abstract
Phthalocyanines and their analogs have practical interest at development devices with low consumption because these materials are known to considerably modify their electrophysical properties on adsorption of the active gases at low temperatures. The conductivity, resistance-temperature relationship, sensor properties relative to NO, O2 and H2S of films based on oligo-(OPc) and polyphthalocyanines (PPc) containing Co, Cu, Fe and Mn were investigated in the present work. Polymeric films were deposited on the test structures with a pair of interdigital metal electrodes. The sensor’s active area was 4.0 × 4.0 mm, and electrode gap was egual 0.08 mm. The sensitive layers were formed by two methods. The soluble OPcs were deposited from their saturated dimethylformamide solutions and insoluble PPcs were deposited by the thermal sputtering in vacuum. The investigations were carried out under the sensor thermal stabilization conditions in the range 50–250 °C with the constant values of the heater resistance.
1. Introduction
The search for new materials with sensitivity to gases at low temperatures remains an urgent task. This factor is important for portable devices as they operate at low consumption. In this case the sensor should be as sensitive as possible with the lowest operating temperature. A sensitive layer’s conductivity of such sensor has to change with a concentration of an active gas by the value that is large enough to be measured by usual methods. These measurements should be reversible and stable in time.
Organic semiconductive materials are known to considerably modify their electrophysical properties on adsorption of the active gases at low temperatures. These changes are generally take place due to the formation of the complexes with the charge transfer at the donor-acceptor interaction of the sensitive layer with the determined substance. It is confirmed by high adsorptive and electrophysical activity of phthalocyanines and their analogs, which contain azaporphines macrocycles with the developed system of conjugated double bonds [1,2,3,4]. Introducing metal into the macrocycle’s “window” of phthalocyanine is accompanied by the change of the electron density at the peripheral nitrogen atoms and, consequently, the change of the properties of substance. Despite the structure of the elementary polyphthalocyanine element being similar to the phthalocyanine structure, polyphthalocyanine has a more developed interface system that increases its nucleophilic properties. Besides, in polymer metal can additionally be linked to the framing and end macromolecule groups while in a phthalocyanine metal can be located only at one position. Hereby, depending on the metal properties, the nucleophilic properties of the polymer can be both increased and diminished. Such features of polyphthalocyanines can be used at development of sensitive layers for chemical sensors and devices with low consumption.
2. Materials and Methods
The substances (Figure 1) were synthesized by polycyclotetramerization of tetranitrile of pyromellitic acid in bulk and in polar solvents at 180–300 °C for 5–30 h in the presence of 0–5 mol. % carbamide.
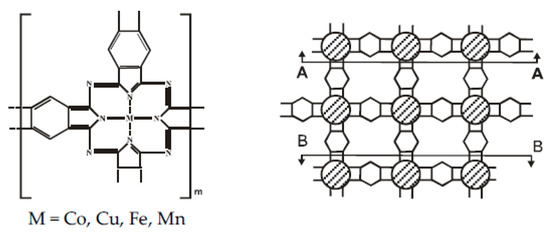
Figure 1.
The structure polyphthalocyanine (oligophthalocyanine inside AA-BB).
Polymeric films were deposited on the test structures with a pair of interdigital metal electrodes formed at their surface. The sensor’s active area was 4.0 × 4.0 mm, and electrode gap was egual 0.08 mm. The sensitive layers were formed by two methods. The soluble OPcs were deposited from their saturated dimethylformamide solutions and insoluble PPcs were deposited by the thermal sputtering in vacuum. The films thermal sputtering was obtained at evaporation temperature of 300–1000 °C and pressure in chamber 1.33 × 10−8 bar. After being deposited on a test structure the films were annealed in air at a temperature of 250 °C to stabilize their electrophysical properties.
The films’ properties were examined on sample gas mixtures using a dynamic blender “Environics-4000” (Environics, Tolland, CT, USA) and Dräger test ampoules (Dräger, Lübeck, Germany). The investigations were carried out under the sensor thermal stabilization conditions in the range 50–250 °C with the constant values of the heater resistance and applied power.
3. Results and Discussion
The metal content in the obtained polymers was from 0.5 to 15 wt % according to X-ray fluorescence analysis.
The conductivity of the films, their resistance-temperature relationship, sensor properties relative to nitric oxide, oxygen and hydrogen sulfide as well as effect of the conditions of film formation on their characteristics was study. The sensitive layers’ characteristics of changed with temperature of the film in different ways (Figure 2). It was shown that preliminary temperature treatment significantly affects adsorption activity of polymeric film. The sensitive layers’ characteristics changed with temperature of film in different ways that connected with composition and structure of polymeric phthalocyanine.
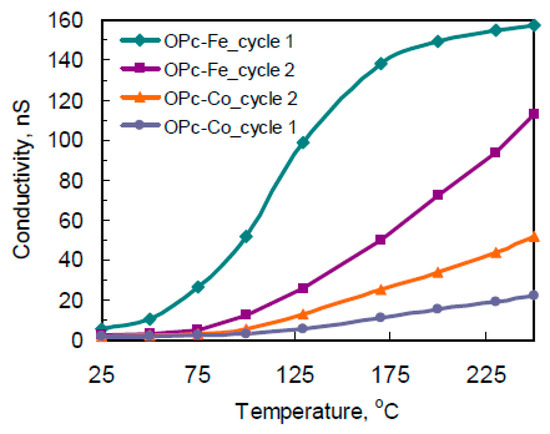
Figure 2.
Typical conductivity dependence on temperature for OPc-Co and OPc-Fe films at two serial cycles.
The presence of the conjugation system in phthalocyanines and their analogs is known to promotes not only the appearance of particular physical properties, but also defines the specific behavior in the some chemical reactions, for example, the formation of transfer charge complex with electroacceptor compounds. Thus, the interaction of polyphthalocyanines with gas molecules is complex, also determined by the presence of the metal type and its quantity, which also makes a significant contribution to the adsorption behavior of the polymer film.
Films showed different sensitivity to presence of NO in range of 0–500 ppb in air. The resistance of polymeric film with Cu decreases up to 3 times (Figure 3).
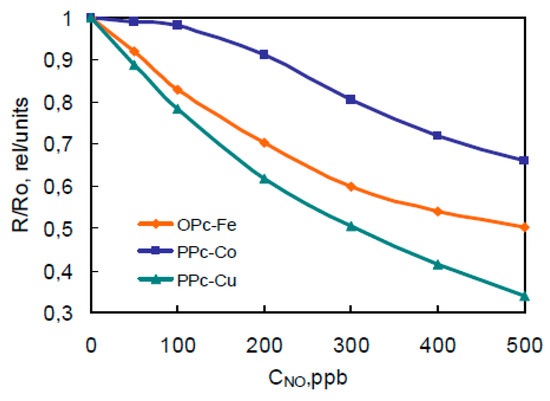
Figure 3.
Typical resistance dependence on NO concentration in air at 100 °C.
The content of oxygen in measured medium has different influence on electrophysical behavior of films (Figure 4). In case of OPc-Cu the resistance falls up to 2 times, and at that time for PPc-Cu films this change is smaller.
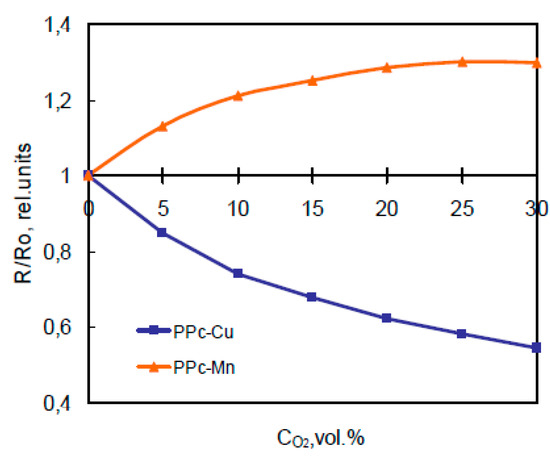
Figure 4.
Typical resistance dependence on oxygen concentration for films at 200 °C.
The conductivity of PPc-Cu decreases up to 10 times at 100–150 °C in air medium with H2S of 0.1 ppm, while time constant does not exceed 60 s.
Despite the structure of the elementary polyphthalocyanine element is similar to the phthalocyanine structure, it exhibits a more developed conjugation system that amplifies nucleophilic properties of polyphthalocyanines. At this metal can be in different positions of the polymer that results in different electronic structures of polyphthalocyanines and affect their adsorption behaviour, which has important role for sensitive layer’s working in sensor. The metal type and content in polymeric phthalocyanine affects the electophysical characteristics and behavior at interaction with gas molecules.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fitl, P.; Vrnata, M.; Kopecky, D.; Vlcek, J.; Skodova, J.; Hofmann, J.; Myslik, V. Laser deposition of tetrasulfonated phthalocyanine layers for gas sensors. Adv. Mater. Phys. Chem. 2012, 2, 84–88. [Google Scholar] [CrossRef]
- Rivera, M.; Reyes, B.; Sanchez-Vergara, M.E.; Mendoza-Huizar, L.H. Conductive behavior and morphology of axially modified gallium phthalocyanine thin films onto indium tin oxide substrates. Adv. Mater. Phys. Chem. 2016, 6, 211–219. [Google Scholar] [CrossRef]
- Shu, J.H.; Wikle, H.C.; Chin, B.A. Passive chemiresistor sensor based on iron (II) phthalocyanine thin films for monitoring of nitrogen dioxide. Sens. Actuators B-Chem. 2010, 148, 489–503. [Google Scholar] [CrossRef]
- Sizun, T.; Bouvet, M.; Chen, Y.; Barochi, G.; Rossignol, J. Differential study of substituted and unsubstituted cobalt phthalocyanines for gas sensor applications. Sens. Actuators B-Chem. 2011, 159, 163–170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).