Commercially Fabricated Printed Circuit Board Sensing Electrodes for Biomarker Electrochemical Detection: The Importance of Electrode Surface Characteristics in Sensor Performance †
Abstract
:1. Introduction
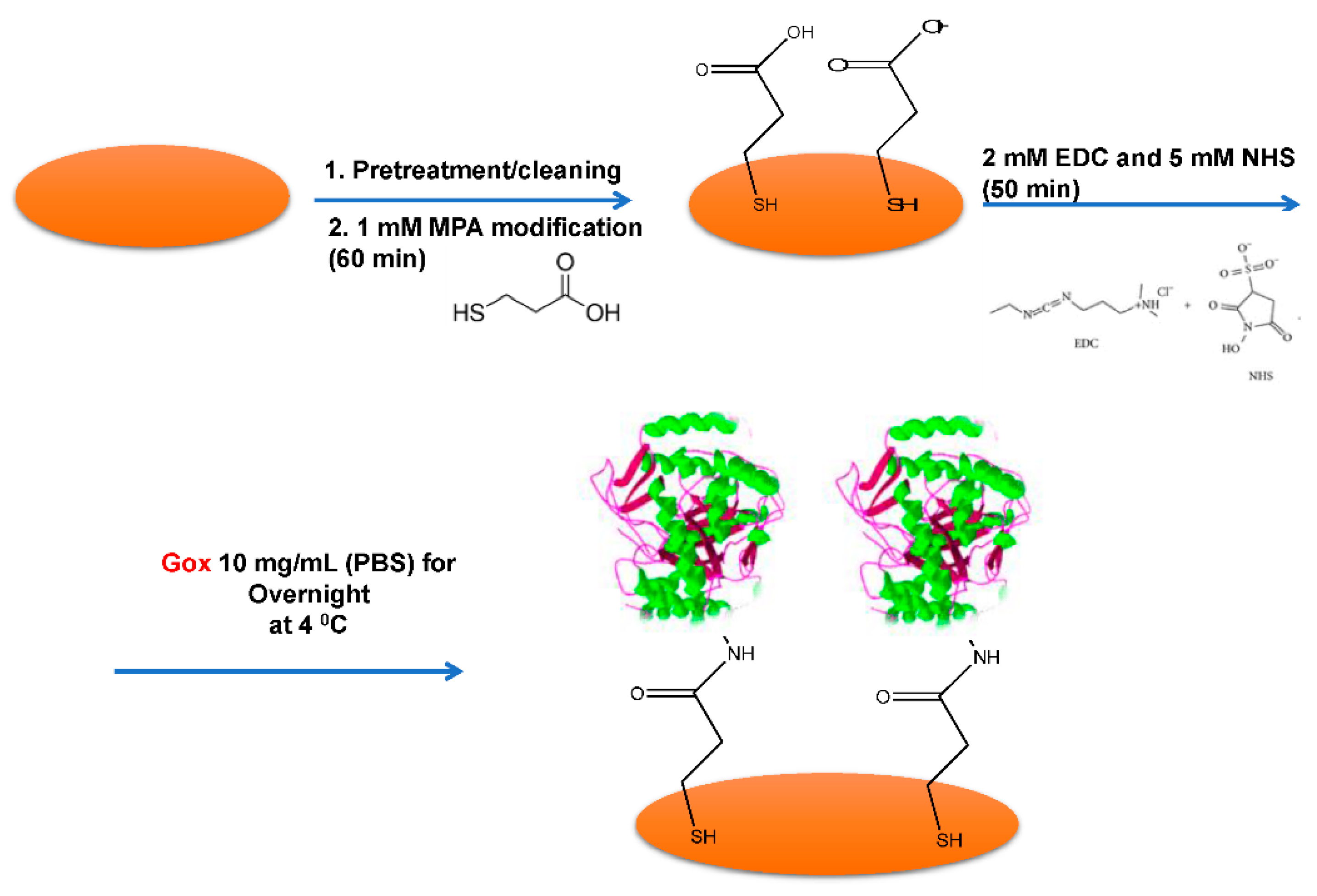
2. Materials and Methods
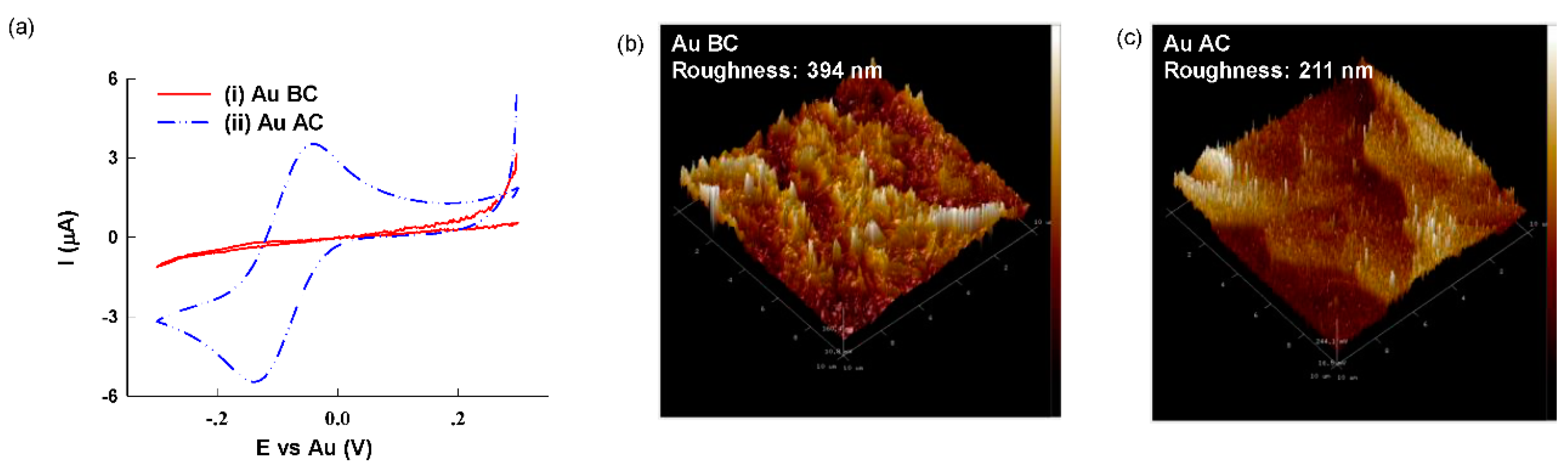
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Kesherwani, R.; Tiwari, A.K.; Kumar, D. A review on diabetes mellitus. Pharma Innov. J. 2016, 5, 36–40. [Google Scholar]
- Available online: https://www.idf.org/our-network/regions-members/europe/members/163-turkey.html (accessed on 26 November 2018).
- Moschou, D.; Tserepi, A. The lab-on-PCB approach: Tackling the μTAS commercial upscaling bottleneck. Lab Chip 2017, 17, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Moschou, D.; Greathead, L.; Pantelidis, P.; Kelleher, P.; Morgan, H.; Prodromakis, T. Amperometric IFN-γ immunosensors with commercially fabricated PCB sensing electrodes. Biosens. Bioelectron. 2016, 86, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.T.C.; Ferreira, M.J.M.S.; Puga, J.R.T.; Sales, M.G.F. Screen-printed electrode produced by printed-circuit board technology. Application to cancer biomarker detection by means of plastic antibody as sensing material. Sens. Actuators B Chem. 2016, 223, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.L.; Henry, O.Y.; Joda, H.; Solnestam, B.W.; Kvastad, L.; Johansson, E.; Akan, P.; Lundeberg, J.; Lladach, N.; Ramakrishnan, D.; et al. Multiplex PCB-based electrochemical detection of cancer biomarkers using MLPA-barcode approach. Biosens. Bioelectron. 2016, 82, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.; Henry, O.Y.; Dhaenens, K.; Acero Sanchez, J.L.; Gielen, A.; Werne Solnestam, B.; Lundeberg, J.; O’Sullivan, C.K.; Vanfleteren, J. Fabrication and functionalization of PCB gold electrodes suitable for DNA-based electrochemical sensing. Bio-Med. Mater. Eng. 2014, 24, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, H.; Cheng, C.; Wu, J.; Chen, J.; Eda, S.; Aghdam, E.N.; Ghavifekr, H.B. A highly sensitive and specific capacitive aptasensor for rapid and label-free trace analysis of Bisphenol A (BPA) in canned foods. Biosens. Bioelectron. 2017, 89, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.R.; Muthukumar, S.; Chaudhry, S.; Anguiano, J.; Prasad, S. Ultrasensitive nanostructure sensor arrays on flexible substrates for multiplexed and simultaneous electrochemical detection of a panel of cardiac biomarkers. Biosens. Bioelectron. 2017, 89, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zang, J.; Liu, Y.; Lu, Z.; Li, Q.; Li, C.M. Simultaneous detection of lactate and glucose by integrated printed circuit board based array sensing chip. Anal. Chim. Acta 2013, 771, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Moschou, D.; Morgan, H.; Prodromakis, T. A PCB-based electrochemical glucose biosensing platform. In Proceedings of the 2016 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences (microTAS 2016), Dublin, Ireland, 9–13 October 2016; pp. 1047–1048. [Google Scholar]
- Pu, Z.; Wang, R.; Wu, J.; Yu, H.; Xu, K.; Li, D. A flexible electrochemical glucose sensor with composite nanostructured surface of the working electrode. Sens. Actuators B Chem. 2016, 230, 801–809. [Google Scholar] [CrossRef]
- Jamaluddin, A.; Taufik, U.; Iriani, Y.; Budiawanti, S. Suyitno Simple fabricating PCB-based inter digital capacitor for glucose biosensor. Available online: https://aip.scitation.org/doi/abs/10.1063/1.4968345 (accessed on 28 November 2018).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, G.; Regoutz, A.; Moschou, D. Commercially Fabricated Printed Circuit Board Sensing Electrodes for Biomarker Electrochemical Detection: The Importance of Electrode Surface Characteristics in Sensor Performance. Proceedings 2018, 2, 741. https://doi.org/10.3390/proceedings2130741
Dutta G, Regoutz A, Moschou D. Commercially Fabricated Printed Circuit Board Sensing Electrodes for Biomarker Electrochemical Detection: The Importance of Electrode Surface Characteristics in Sensor Performance. Proceedings. 2018; 2(13):741. https://doi.org/10.3390/proceedings2130741
Chicago/Turabian StyleDutta, Gorachand, Anna Regoutz, and Despina Moschou. 2018. "Commercially Fabricated Printed Circuit Board Sensing Electrodes for Biomarker Electrochemical Detection: The Importance of Electrode Surface Characteristics in Sensor Performance" Proceedings 2, no. 13: 741. https://doi.org/10.3390/proceedings2130741
APA StyleDutta, G., Regoutz, A., & Moschou, D. (2018). Commercially Fabricated Printed Circuit Board Sensing Electrodes for Biomarker Electrochemical Detection: The Importance of Electrode Surface Characteristics in Sensor Performance. Proceedings, 2(13), 741. https://doi.org/10.3390/proceedings2130741



