1. Introduction
Mercury is one of the most toxic elements able to cause severe acute and chronic problems to the human health (affecting the nervous, cardiovascular, immune and reproductive systems) and the ecosystem [
1,
2]. Considering the importance of mercury monitoring in various samples and the low tolerances, sensitive analytical methodologies are necessary. The most widely used analytical methods for mercury determination are based on atomic absorption and emission spectroscopy but these approaches require expensive instrumentation, trained personnel, laborious sample preparation and are not suitable for on-site analysis [
3,
4].
On the other hand, voltammetric techniques require only portable and inexpensive equipment and are well suited to field analysis. It is generally acknowledged that Au is the best working electrode material for the voltammetric detection of Hg(II) [
4,
5,
6]. Hg(II) can be readily preconcentrated on Au surfaces thanks to the effect of underpotential deposition and, therefore, can be readily detected by anodic stripping voltammetry (ASV). ASV is a two-step electrochemical technique; the initial step involves preconcentration-accumulation by reduction of Hg(II) on the Au working electrode surface with the formation of a Hg(Au) amalgam:
The second step involves oxidation of the accumulated Hg in the amalgam by scanning the potential of the Au working electrode:
During the voltammetric scan, the current-potential (i-E) response is recorded and the oxidation current is used to quantify the Hg(II) concentration in the sample.
Several types of Au-based sensors have been developed for the trace determination of Hg(II) using voltammetric techniques [
4,
5,
6]. Among them, Au disk electrodes, Au (micro)wires, screen-printed electrodes bulk- or surface-modified with Au and CD-based electrodes, are the commonest.
This work describes a novel microfabricated Au-film sensor for the anodic stripping voltammetric determination of Hg(II) at trace levels. The fabrication approach combines a sputtering process for the deposition of a 100 nm-thick Au film on an oxidized silicon wafer and photolithography for the definition of sensor geometry.
2. Experimental
2.1. Chemicals andReagents
All the chemicals were of analytical reagent grade and purchased from Sigma-Aldrich (Greece) unless stated otherwise. Working 2.0 and 10.0 mg L−1 standard solutions of Hg(II) were prepared from a 1000 mg L−1 standard Hg(II) solution. A 0.10 moL L−1 solution of HCl was prepared by appropriate dilution of 30% (w/w) HCl.
2.2. Microfabricated Au-FilmSensor
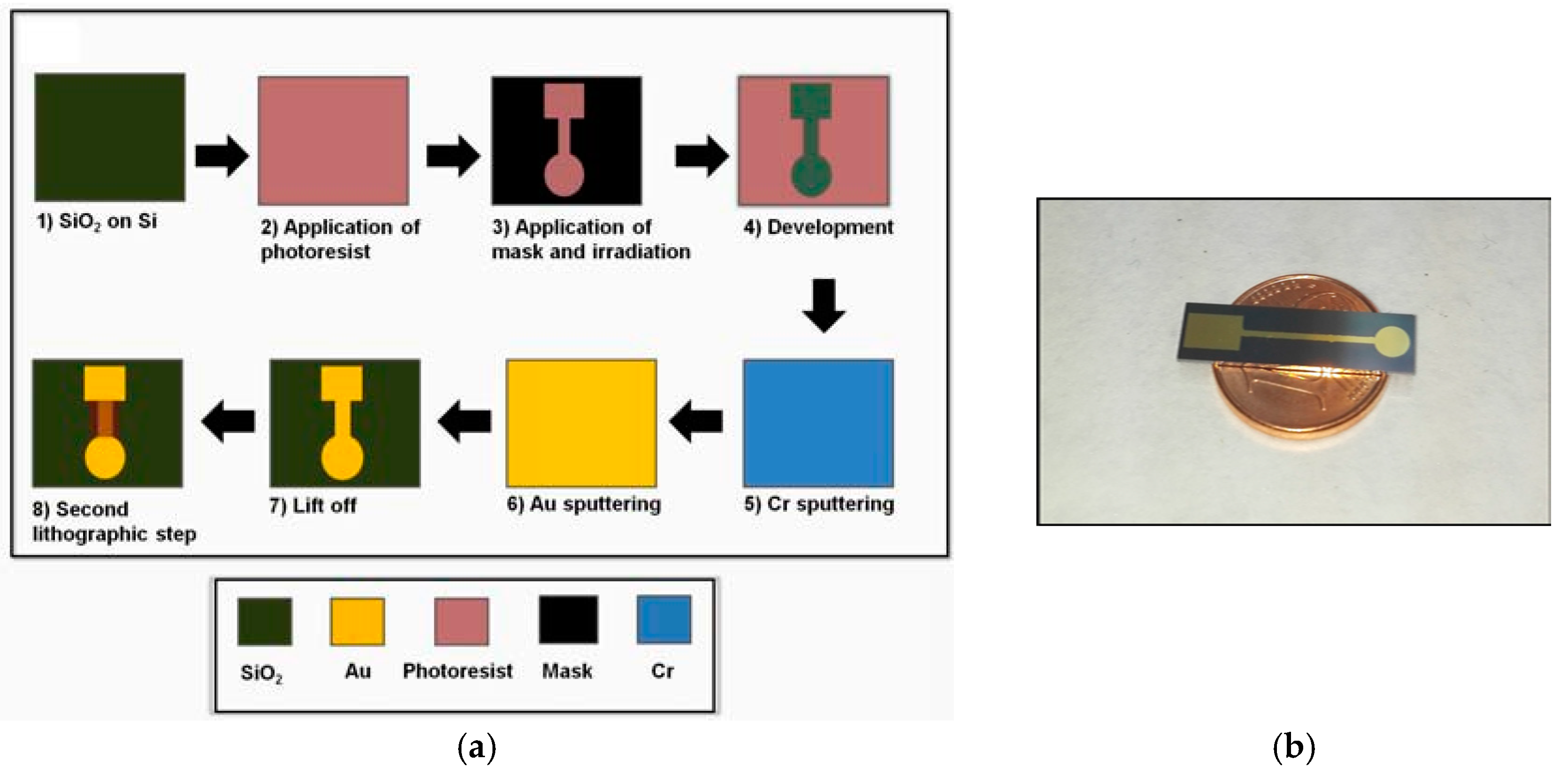
The process for the fabrication of the Au-film sensors is schematically illustrated in
Figure 1a. Silicon wafers were covered with a layer of SiO
2 1080 nm thick by means of wet thermal oxidation. The wafer was spin-coated with a layer of AZ5214 photoresist and the shape of the electrodes was defined by photolithography. Then, Cr and Au were sputtered on the wafer at a nominal thickness of 5 and 100 nm, respectively. The electrodes were patterned by a lift-off process of the polymer. A second photolothographic step was used to define the sensing area and to isolate it from the grip area. A photograph of the sensor is illustrated in
Figure 1b.
2.3. Instrumentation
For electrochemical measurements, a portable USB–powered EMSTAT potentiostat (Palmsens, Utrecht, The Netherlands) was used in combination with the PSTrace 4.2 software. The microfabricated Au-film working electrode was used in a three–electrode configuration combined with a Ag/AgCl (sat. KCl) reference electrode and a Pt wire as the counter electrode. A magnetic bar rotated at approx. 1000 rpm provided stirring.
2.4. ExperimentalProcedure
For the voltammetric experiments, 10.0 mL of 0.10 mol L−1HCl was placed in the cell and the background voltammogram was recorded using the conditions detailed in the next paragraph. Additions of a Hg(II) standard were made in the cell and the analysis procedure was repeated as required.
The parameters for the cyclic voltammetry experiments were as follows: scanrate,0.1Vs−1; step, 2 mV; initial potential, 0.00 V; final potential, +0.80 V vs. Ag/AgCl. The parameters for the square wave anodic stripping voltammetry experiments were: preconcentration time, 120 s; preconcentration potential, −0.20 V; frequency, 25 Hz; step, 4 mV; pulse amplitude, 25 mV; initial potential, +0.00 V; final potential, +0.70 V vs. Ag/AgCl.
3. Results and Discussion
Initial characterization of the electrode was performed by cyclic voltammetry in the potential range 0.00V to +0.80V in 0.10mol L
−1 HCl containing 150μgL
−1 Hg(ΙΙ) after preconcentration at −0.20 V for 30 s (
Figure 2). During the forward anodic scan, a prominent and well-defined oxidation peak appears at +0.56 V due to the oxidation of the accumulated Hg. During the reverse cathodic scan, a weak reduction peak appears at +0.52 V due to the reduction of Hg(II) generated during the anodic scan and existing at the vicinity of the electrode. The cyclic voltammetry experiment suggests that the proposed sensor is suitable for Hg(II)determination.
After a detailed study of the preconcentration time, the preconcentration potential and the composition of supporting electrolyte, the selected conditions were: 120 s, −0.20 V and 0.10 mol L−1HCl, respectively. The potential interference by various metal ions (Pb(II), Cd(II), Zn(II), Sn(II), Sb(III), Bi(III), Cu(II)) was investigated; only Cu(II) produced a stripping peak at +0.30 V, which was well separated from the Hg peak.
Quantitative analysis was performed with square wave ASV. Representative stripping voltammograms and the respective calibration plot (relating the stripping peak current to the Hg(II) concentration) are illustrated in
Figure 3. The calibration plot was linear in the Hg(II) concentration range examined (10–100 μg L
−1) with good linearity (R
2 > 0.99). The limit of detection, calculated as the Hg(II) concentration that corresponds to a signal-to-noise ratio of 3, was 1.5 μg L
−1. The % relative standard deviation of 10 consecutive measurements at the same sensor was 3.1%.
Besides, the sensors were tested for the voltammetric determination of Hg(II) in a fish oil sample, spiked with 10 μg L−1 of Hg(II). The sample was digested in the presence of a 1:1 HCl (30%)/H2O2 (30%) mixturebefore analysis. The recovery was 98%.
4. Conclusions
It has been shown that the proposed microfabricated Au-film sensors are suitable for trace Hg(II) determination. These sensors exhibit satisfactory analytical features, are easy to fabricate, offer scope for mass-production, do not require any pre-treatment step, are semi-disposable and are suitable for on-site analysis.